Soy-Based Soft Matrices for Encapsulation and Delivery of Hydrophilic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
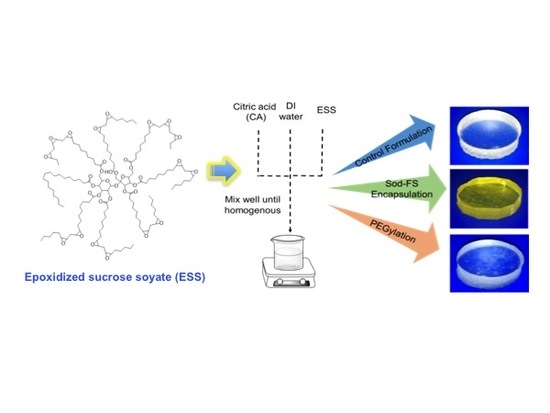
2.2. Preparation of ESS-CA Matrix
2.3. Determination of Loading Content
2.4. Characterization and Measurements
2.4.1. Attenuated Total Reflectance-Infrared (ATR-IR) Spectroscopy
2.4.2. Gel Permeation Chromatography
2.4.3. Viscoelasticity TESTING
2.4.4. Water Contact Angle Measurement
2.4.5. Content Release Studies
2.4.6. Scanning Electron Microscopy (SEM)
2.4.7. Kinetic Analysis of the Release Data of SOD-FS from ESS-Matrices
2.5. Cytotoxicity Studies of ESS
3. Results
3.1. Fabrication of the Matrices
3.2. Surface, Microstrutural and Mechanical Property Analysis of ESS-CA Matrices
3.3. Content Release Studies
3.4. Release Kinetics
3.5. Cytotoxicity Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Cudworth, G. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 1998, 40, 1–12. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef]
- Li, C. Poly(l-glutamic acid)-anticancer drug conjugates. Adv. Drug Deliv. Rev. 2002, 54, 695–713. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444–14451. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Tahara, K.; Yamamoto, K.; Nishihata, T. Overall mechanism behind matrix sustained release (SR) tablets prepared with hydroxypropyl methylcellulose 2910. J. Control. Release 1995, 35, 59–66. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Murthy, T.K.; Pai, M.R.; Mehta, P.R.; Chowdary, P.B. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS PharmSciTech 2003, 4, 18–23. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 38. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.; Bae, Y.H.; Kim, S.W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control. Release 2000, 63, 155–163. [Google Scholar] [CrossRef]
- Natarajan, J.; Rattan, S.; Singh, U.; Madras, G.; Chatterjee, K. Polyanhydrides of castor oil-sebacic acid for controlled release applications. Ind. Eng. Chem. Res. 2014, 53, 7891–7901. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.; Schicke, B.; Müller-Goymann, C. Further characterization of theobroma oil-beeswax admixtures as lipid matrices for improved drug delivery systems. Eur. J. Pharm. Biopharm. 2006, 64, 294–306. [Google Scholar] [CrossRef] [PubMed]
- De, C.; Vervaet, C.; Görtz, J.; Remon, J.P.; Berlo, J. Bioavailability of ibuprofen from matrix mini-tablets based on a mixture of starch and microcrystalline wax. Int. J. Pharm. 2000, 208, 81–86. [Google Scholar]
- Toro-Vazquez, J.; Morales-Rueda, J.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Humberstone, A.J.; Charman, W.N. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv. Drug Deliv. Rev. 1997, 25, 103–128. [Google Scholar] [CrossRef]
- Shogren, R.L.; Petrovic, Z.; Liu, Z.; Erhan, S.Z. Biodegradation behavior of some vegetable oil-based polymers. J. Polym. Environ. 2004, 12, 173–178. [Google Scholar] [CrossRef]
- Chandran, S.; Asghar, L.F.; Mantha, N. Design and evaluation of ethyl cellulose based matrix tablets of ibuprofen with ph modulated release kinetics. Indian J. Pharm. Sci. 2008, 70, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Sun, L.; Wang, P.; Liu, R.; Su, Z.; Zhang, S. Soybean oil-based polyurethane networks as candidate biomaterials: Synthesis and biocompatibility. Eur. J. Lipid Sci. Technol. 2012, 114, 1165–1174. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.J.; Bultema, L.; Eidenschink, N.; Webster, D.C. Bio-based high functionality polyols and their use in 1K polyurethane coatings. J. Renew. Mater. 2013, 1, 141–153. [Google Scholar] [CrossRef]
- Guo, A.; Demydov, D.; Zhang, W.; Petrovic, Z.S. Polyols and polyurethanes from hydroformylation of soybean oil. J. Polym. Environ. 2002, 10, 49–52. [Google Scholar] [CrossRef]
- List, G.; Neff, W.; Holliday, R.; King, J.; Holser, R. Hydrogenation of soybean oil triglycerides: Effect of pressure on selectivity. J. Am. Oil Chem. Soc. 2000, 77, 311–314. [Google Scholar] [CrossRef]
- Pan, X.; Sengupta, P.; Webster, D.C. High biobased content epoxy-anhydride thermosets from epoxidized sucrose esters of fatty acids. Biomacromolecules 2011, 12, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Webster, D.C. Naturally occurring acids as cross-linkers to yield voc-free, high-performance, fully bio-based, degradable thermosets. Macromolecules 2015, 48, 7127–7137. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Poly(ether urethane) networks from renewable resources as candidate biomaterials: Synthesis and characterization. Biomacromolecules 2007, 8, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Kolanthai, E.; Sarkar, K.; Meka, S.R.K.; Madras, G.; Chatterjee, K. Copolyesters from soybean oil for use as resorbable biomaterials. ACS Sustain. Chem. Eng. 2015, 3, 880–891. [Google Scholar] [CrossRef]
- Yang, J.; Webb, A.R.; Pickerill, S.J.; Hageman, G.; Ameer, G.A. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials 2006, 27, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Webb, A.R.; Ameer, G.A. Novel citric acid-based biodegradable elastomers for tissue engineering. Adv. Mater. 2004, 16, 511–516. [Google Scholar] [CrossRef]
- Reza, M.S.; Quadir, M.A.; Haider, S.S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J. Pharm. Pharm. Sci. 2003, 6, 282–291. [Google Scholar] [PubMed]
- Hakim, M.; Jalil, R. Evaluation of a New Rate Retarding Polymer Kollidon SR as Matrix Tablets. Master’s Thesis, University of Dhaka, Dhaka, Bangladesh, 2001. [Google Scholar]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Göpferich, A. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 2001, 48, 229–247. [Google Scholar] [CrossRef]
- Möckel, J.E.; Lippold, B.C. Zero-order drug release from hydrocolloid matrices. Pharm. Res. 1993, 10, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Sengupta, P.; Webster, D.C. Novel biobased epoxy compounds: Epoxidized sucrose esters of fatty acids. Green Chem. 2011, 13, 965–975. [Google Scholar] [CrossRef]
- Dakkuri, A.; Schroeder, H.G.; Deluca, P.P. Sustained release from inert wax matrixes II: Effect of surfactants on tripelennamine hydrochloride release. J. Pharm. Sci. 1978, 67, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Lordi, N.G. Sustained release dosage forms. Theory Pract. Ind. Pharm. 1986, 3, 430–456. [Google Scholar]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B.; Simonelli, A.P.; Higuchi, W.I. Drug release from wax matrices I. Analysis of data with first-order kinetics and with the diffusion-controlled model. J. Pharm. Sci. 1968, 57, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.B.; Simonelli, A.P.; Higuchi, W.I. Drug release from wax matrices II. Application of a mixture theory to the sulfanilamide-wax system. J. Pharm. Sci. 1968, 57, 278–282. [Google Scholar] [CrossRef] [PubMed]







| Sample | Molar Ratio | T °C/Time | Matrix Type | ||||
|---|---|---|---|---|---|---|---|
| ESS | Citric Acid | Di-H2O | PEG | Sod-FS | |||
| EC-1 | 1 | 0.98 | 6 | 80/2 h | High [H+] | ||
| EC-2 | 1 | 0.98 | 8 | 80/2 h | Low [H+] | ||
| ECP-750 | 1 | 0.98 | 6 | 0.007 | 80/3 h | PEGylated Matrices | |
| ECP-2k | 1 | 0.98 | 6 | 0.007 | 80/3 h | ||
| ECP-5k-5 | 1 | 0.98 | 6 | 0.007 | 80/3 h | ||
| ECP-5k-1 | 1 | 0.98 | 6 | 0.003 | 80/2.5 h | ||
| EC-Sod-FS-L | 1 | 0.98 | 6 | 0.002 | 80/2.5 h | Low [Sod-FS] | |
| EC-Sod-FS-H | 1 | 0.98 | 6 | 0.005 | 80/2.5 h | High [Sod-FS] | |
| Sample | n | k | r2 |
|---|---|---|---|
| EC-1 | 0.8678 ± 0.0527 | 0.0171 ± 0.0034 | 0.9662 ± 0.0038 |
| ECP-750 | 0.7097 ± 0.0118 | 0.0456 ± 0.0034 | 0.9639 ± 0.0096 |
| ECP-2K | 0.7238 ± 0.0206 | 0.0366 ± 0.0037 | 0.9725 ± 0.0044 |
| ECP-5K-5 | 0.6802 ± 0.0122 | 0.0455 ± 0.0018 | 0.9805 ± 0.0026 |
| ECP-5K-1 | 0.7972 ± 0.0474 | 0.0258 ± 0.0041 | 0.9725 ± 0.0023 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitemere, R.; Stafslien, S.; Jiang, L.; Webster, D.; Quadir, M. Soy-Based Soft Matrices for Encapsulation and Delivery of Hydrophilic Compounds. Polymers 2018, 10, 583. https://doi.org/10.3390/polym10060583
Chitemere R, Stafslien S, Jiang L, Webster D, Quadir M. Soy-Based Soft Matrices for Encapsulation and Delivery of Hydrophilic Compounds. Polymers. 2018; 10(6):583. https://doi.org/10.3390/polym10060583
Chicago/Turabian StyleChitemere, Ruvimbo, Shane Stafslien, Long Jiang, Dean Webster, and Mohiuddin Quadir. 2018. "Soy-Based Soft Matrices for Encapsulation and Delivery of Hydrophilic Compounds" Polymers 10, no. 6: 583. https://doi.org/10.3390/polym10060583
APA StyleChitemere, R., Stafslien, S., Jiang, L., Webster, D., & Quadir, M. (2018). Soy-Based Soft Matrices for Encapsulation and Delivery of Hydrophilic Compounds. Polymers, 10(6), 583. https://doi.org/10.3390/polym10060583