3.2. Silanization Kinetics in Model Olefin Systems
The success of TESPT as a silane coupling agent in silica/rubber systems was due to two main chemical reactions: silanization of the silica and coupling towards the elastomers that finally lead to chemical bridges between the silica surfaces and elastomer molecules. As stated before, the silanization reaction is divided into a primary and secondary reaction (
Figure 3). The primary reaction can proceed via two pathways: either by direct condensation between the silanol groups of the silica surface and one alkoxy group of TESPT, or by hydrolysis of the alkoxy groups of TESPT to form reactive hydroxyl groups prior to the condensation reaction [
14]. Both pathways release EtOH as byproduct. The secondary reaction often occurs between adjacent TESPT molecules on the filler surface, also releasing ethanol.
The EtOH released from the silanization reaction of the silica/silane system in the
n-decane model compounds calculated in relation to the concentration of TESPT used is shown in
Figure 4. The concentration of released EtOH increased with increasing reaction time. In the presence of amines, the ethanol concentration rose sharply within 10 min to exceed 2 moles per mole of TESPT due to the primary reaction, and thereafter rose more slowly. The silica/silane systems with all amine types showed significantly higher concentrations of EtOH than the one without amine due to the enhanced silanization reaction catalyzed by the alkaline amines. Hydrolysis of the silane can readily occur under basic conditions [
19]. Thus, amine provides a catalytic effect on the hydrolysis of alkoxy groups in the silane molecules to form reactive hydroxyl moieties prior to the condensation reaction [
19,
20]. In the presence of amine and water, a hydroxyl ion (OH
−) was formed, and EtOH was released from the TESPT molecules via a pentacoordinate intermediate, forming Si-OH [
19,
20]. Subsequently, the amines catalyzed the condensation reaction between the Si-OH on the silica surface and the alkoxy groups in the TESPT molecules [
21,
22,
23]. The catalyzed condensation reaction occured after the amine entered into interaction with the silanol group on the silica surface via hydrogen bonding, enhancing more nucleophilicity on the silica surface. Thus, the silica surface could interact easier with the silicon atom in the TESPT molecule that led to the condensation reaction [
21]. This role of amine as a catalyst for the silanization reaction is depicted in
Figure 5.
In case of the primary silanization reaction, 1 mol of TESPT which reacted with silanol groups on the silica surface released 2 mols of EtOH as byproduct. The rate constant of the silanization reaction depended on the TESPT concentration, which can be described by a first order rate law. Based on the concentration of TESPT and released EtOH, the rate of the silanization reaction can be calculated using Equation (3) as shown below [
14].
where [
TESPT] is concentration of TESPT; [
EtOH] is a concentration of EtOH;
ka is rate constant of primary silanization reaction, and
t is time.
Based on Equation (3) and the model reaction procedure as laid out in
Section 2.3, the rate constant of the primary silanization reaction can be calculated in the usual manner by plotting ln [TESPT]
t − ln [TESPT]
0 against time
t; where [TESPT]
t is the TESPT concentration at time
t and [TESPT]
0 is the initial TESPT concentration. The slope of the plot is taken as the reaction rate constant.
Figure 6 shows the rate constants of the primary silanization reaction of silica/TESPT with different amine types in comparison with the reference without amine. It shows that the reaction mixture of silica/TESPT without amine displayed the lowest rate constant of the primary silanization reaction, equal to 0.039 min
−1. The reference with DPG had a primary silanization reaction rate constant of 0.139 min
−1, where the rate constants for the systems with alternative amines were in the range of 0.070 to 0.143 min
−1. This demonstrates indeed that the use of amines promotes the primary silanization reaction 1.8 to 3.7 times.
The different amine types with similar pKa values but different structures showed variation in the rate constant of the primary silanization reaction, as shown in
Figure 6. The amines with linear aliphatic chains (i.e., HEX, DEC, and OCT) showed higher rate constants compared with the amines with a cyclic structure (i.e., CYC, DIC, and QUI). Of the primary amines with linear alkyl chains with the best catalytic effect, HEX (with the shortest alkyl chain) gave the highest rate constant of 0.143 min
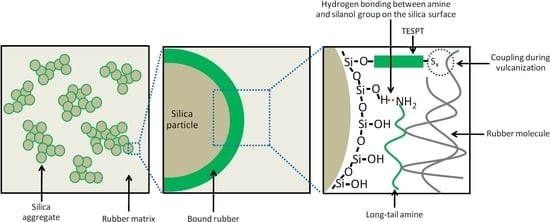
−1. Longer alkyl chains of the aliphatic amines apparently decreased the reaction rate and the presence of a cyclic aliphatic structure further reduced the rate. The bi-cyclic structure of DIC resulted in the lowest rate constant. The difference in promoting the silanization by amines having similar pKa values in the silica/silane system may be attributed to their different adsorption efficiency of the amine molecules on the silica surface. The cyclic ring hampered the adsorption on the silica surface due to steric hindrance, while straight alkyl-chains allowed for easy access to the silanol groups on the silica surface. The increase of one cyclic ring in CYC, to two cyclic rings in DIC, drastically reduced the primary silanization reaction rate by 39%, whereas the increase of the linear aliphatic chain length from C6 to C10 and C18, decreased the reaction rate constants by only about 18% and 20%, respectively. The adsorption of amines with the long aliphatic chains from C10 to C18 on the silica surface introduced slightly more steric effects that may interfere with the silanization reaction. When compared with the short chain amine, the long tail of the amines could shield the silica surface and deactivate the free silanol groups, as displayed in
Figure 7.
The secondary silanization reaction that takes place between the left-over alkoxy groups in adjacent silane molecules bound on the silica surface had a much lower rate constant compared to the primary reaction. The data are in agreement with the rate constants reported by Görl et al. [
14] for the silica/TESPT system in an
n-decane model reaction system, in which at 140 °C the primary vs. secondary reaction rate constants were found as 0.122 vs. 0.008 min
−1, respectively. The rate constants of the secondary silanization reaction of the silica/silane mixture is also shown in
Figure 6, in which all mixtures with amines show higher rate constants than the mix without amine. However, the different amine structures had only a small influence on the rate constant of this secondary silanization reaction, found in the range of 0.01 to 0.02 min
−1. This very low or almost negligible secondary reaction may be attributed to the limited number of silanes grafted on adjacent isolated silanol sites, as a minimum distance between the two SiOH groups of greater than 0.4 nm is estimated in order to be accessible for the incoming silane molecules [
16].
As displayed in
Figure 6, among the amine types studied, HEX gave the highest rate constant of the primary silanization reaction, slightly higher than that of the reference mixture of silica/silane with DPG. Hexylamine is a primary amine with C6 aliphatic tail, while DPG is a secondary amine with aromatic rings, but containing 3 polar -NH groups. These two types of amines were further studied for the effect of the amount on the primary silanization reaction rate constant (
Figure 8).
Both DPG and HEX showed the same trend of changes in the rate constants of the primary silanization reaction against the molar quantities used in the model system. With increasing amine loading till 0.2 mmol, the rate constant increased, and thereafter decreased slightly, and tended to level off at an excessive amine loading, as shown in
Figure 8. The presence of amine in the silica/silane model system promoted the rate constant of the primary silanization reaction about three fold. The decrease of the silanization reaction rate constant with increasing amounts of amines to more than 0.2 mmol was due to amine adsorption on the silica surface via hydrogen bonding between the amino groups in the amine molecule and the silanol groups on the silica surface causing reduced accessibility for the silane molecules.
3.3. Filler-Elastomer Interactions and Interfacial Compatibility as Enhanced by the Silanization Reaction in Practical Silica-Reinforced NR Compounds
The strong silica-elastomer interaction in silica/silane-reinforced rubber compounds was obtained via chemical bonding by silane bridge formation. By the efficient use of silane in the silica-reinforced rubber compounds, filler-elastomer interaction was enhanced, and filler-filler interaction could be suppressed. The level of storage modulus at low strain of the filled-rubber compounds indicates stiffness that was raised by the structures of both filler- and rubber-networks. There are four contributions to the storage modulus of the filled rubber compounds: rubber network, hydrodynamic effect, filler-elastomer interaction, and filler-filler interaction. Only the latter was strongly strain-dependent in dependence of the degree of filler-filler interaction. To evaluate the extent of filler-filler interaction or filler networking, the difference in storage modulus at low and high strains, the so-called Payne effect [
10] of the uncured silica-reinforced rubber compounds tested under dynamic conditions, could be determined. A decrease of storage modulus with increasing strain for silica-reinforced rubber compounds was due to filler network breakdown, weakening of hydrogen bonding between adjacent silica aggregates, and slip of the rubber chains on the filler surface. As discussed previously for the role of amines in the silica-reinforced rubber compounds, by a combination of an enhanced silanization reaction, deactivation of free silanol groups left over after the silanization and shielding by the aliphatic chains of the amines, a lower storage modulus at low strain and Payne effect was achieved in the amine-containing compounds, when compared to the one without, as shown in
Figure 9. The rubber compounds with DEC, OCT, and QUI showed similar levels of the filler-filler interactions compared with the mix with DPG. Among the different amine types, the rubber compound with DIC had the lowest rate constant of the primary silanization reaction (
Figure 6), and clearly showed the highest storage modulus and Payne effect (
Figure 9). The steric hindrance introduced by the two cyclic rings of DIC hinders the silane molecules to react towards the silica surface, and gives low accessibility towards the left-over free silanol groups after the silanization reaction. For linear aliphatic amines, the levels of filler-filler interactions of the silica-reinforced NR compounds tended to decrease with increasing chain length, because of enhanced hydrophobicity of the silica surface. In order to emphasize the fact that the Payne effect here was strongly related to the filler-filler network, the storage modulus at 0.56% strain and the G’(0.56%)–G’(100%) for a formulation without any filler present, has also been included in
Figure 9. The absence of filler clearly lowers the G’(0.56%) tremendously relative to the filled compounds, and the G’(0.56%)–G’(100%) of 40 kPa was due to neo-Hookean behavior of the elastomer per se, and was minimal compared to the Payne effects observed for the filled compounds.
Another indicator that can be applied to verify the extent of the filler-elastomer interactions in the present work was the change of heat capacity within the rubber compounds as determined by the DSC technique. As the thermal properties of silica-reinforced rubber compounds change with different silica-elastomer interactions, the heat capacity increment (Δ
Cp) at
Tg, which is related to the fraction of elastomer molecules that change in mobility [
12,
13], can be used to evaluate the interfacial interaction between filler and elastomer. The elastomer chains on the filler surface that cannot move are often called the immobilized polymer layer, indicating the interfacial compatibility of the silica and rubber phases. The Δ
Cp values of silica-reinforced NR compounds with different amine types are shown in
Figure 10. The use of all amines in the silica-reinforced rubber compounds gives lower Δ
Cp than the compound without amine, confirming the enhanced interfacial interactions by the various roles of amines, as previously discussed. The rubber compound with OCT, which showed the lowest Payne effect, exhibited the lowest Δ
Cp when compared to the other compounds. The Δ
Cp values further tended to decrease with increasing length of the alkyl chains, as observed by a reduction of Δ
Cp in the rubber compounds with HEX, DEC, and OCT, respectively. This evidence supports the concept that the tails of the amines could cover the silica surface to enhance hydrophobicity and thus promote silica-elastomer compatibility. In the case of the QUI-containing rubber compound, because of its highest p
Ka value that could also enhance pre-mature coupling between silane and elastomer in addition to the silanization reaction, the immobilized layer of elastomer molecules on the silica surface was increased, and thus, reduced the Δ
Cp.
For the silica-reinforced rubber compounds, the dispersed silica aggregates in the rubber matrix can again re-agglomerate under heat treatment due to the highly polar functional groups on the silica surface. This phenomenon is so-called flocculation. The flocculation rate constant, which indicates how fast re-agglomeration of silica will take place in the rubber matrix [
11] in the beginning phase or scorch period prior to vulcanization of the silica-reinforced NR compounds with different amine types, is shown in
Figure 11. The presence of amines in the silica/silane system, except for the use of DIC, resulted in a lower flocculation rate constant compared to the compound without amine. Diphenyl guanidine and HEX that gave similar rate constants of the primary silanization reaction in the model systems exhibited the same flocculation rate constants as a result of the enhanced silanization by the amines. Among the linear aliphatic amines, the rubber compound with HEX showed a lower flocculation rate constant than with DEC and OCT. The compounds containing the latter two amines with longer alkyl chains could flocculate faster compared to HEX, which gave a greater extent of the silanization reaction. The physical interactions were diminished at elevated temperature, while the chemical interactions remained. Flocculation of dispersed silica in the rubber matrix could still take place after the disappearance of the silica-amine interaction during thermal treatment. The amine with the lowest primary silanization reaction rate constant (i.e., DIC with two steric cyclic rings) (
Figure 6), displayed the highest flocculation rate constant (
Figure 11) in correspondence with its highest Payne effect (
Figure 9). The results demonstrate that the study on the silica/silane model systems and the practical silica-reinforced rubber compounds compared well with each other.