Synthetic Glycopolypeptide Micelle for Targeted Drug Delivery to Hepatic Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Preparation and Characterization of Loading GPM Micelles
3.2. Cell Internalization and Cell Proliferation Inhibition
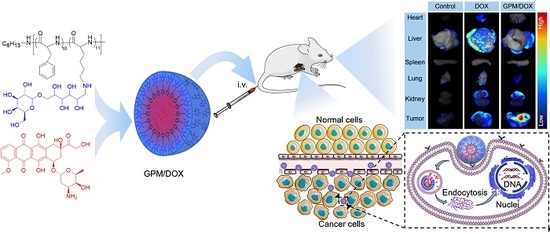
3.3. Biodistribution
3.4. In Vivo Antitumor Efficacy
3.5. In Vivo Antitumor Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, M.; Faraji, A.; Zare, M.; Dolati, P.; Ataei, M.; Manshadi, H.R.D. Molecular Mechanisms of Cardiotoxicity: A Review on the Major Side-effects of Doxorubicin. Indian J. Pharm. Sci. 2017, 79, 335–344. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.J.; Ding, J.X.; Wang, Y.C.; Cheng, J.J.; Ji, S.X.; Zhuang, X.L.; Chen, X.S. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Khawar, I.A.; Kim, J.H.; Kuh, H.-J. Improving drug delivery to solid tumors: Priming the tumor microenvironment. J. Control. Release 2015, 201, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhu, M.; Linhart, H.; Hadaschik, B.; Holland-Letz, T.; Giesel, F.; Kratochwil, C.; Haufe, S. PET imaging with a [68 Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Yu, H.-J.; Liu, X.-W.; Zhang, D.-D.; Li, Z. New Progress of Glycoprotein Glycans Associated with Gastric Cancer. Prog. Biochem. Biophys. 2016, 43, 449–460. [Google Scholar] [CrossRef]
- Pan, S.; Brentnall, T.A.; Chen, R. Glycoproteins and glycoproteomics in pancreatic cancer. World J. Gastroenterol. 2016, 22, 9288–9299. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Jantscher-Krenn, E. Structure-Function Relationships of Human Milk Oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.P.M.; Goncalves, M.P.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Ding, J.; Xiao, C.; Li, L.; Zhuang, X.; Chen, X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Pati, D.; Das, S.; Patil, N.G.; Parekh, N.; Anjum, D.H.; Dhaware, V.; Ambade, A.V.; Sen Gupta, S. Tunable Nanocarrier Morphologies from Glycopolypeptide-Based Amphiphilic Biocompatible Star Copolymers and Their Carbohydrate Specific Intracellular Delivery. Biomacromolecules 2016, 17, 466–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanhueza, C.A.; Baksh, M.M.; Thuma, B.; Roy, M.D.; Dutta, S.; Preville, C.; Chrunyk, B.A.; Beaumont, K.; Dullea, R.; Ammirati, M.; et al. Efficient Liver Targeting by Polyvalent Display of a Compact Ligand for the Asialoglycoprotein Receptor. J. Am. Chem. Soc. 2017, 139, 3528–3536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, Y.; Han, H.H.; Zang, Y.; Li, J.; He, X.P.; Feringa, B.L.; Tian, H. Remote light-controlled intracellular target recognition by photochromic fluorescent glycoprobes. Nat. Commun. 2017, 8, 987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, L.; Zhang, X.; Zhang, Y.; Liu, H.; Sun, B.; Zhao, L.; Ge, N.; Qian, H.; Yang, Y.; et al. Detection of Circulating Tumor Cells in Hepatocellular Carcinoma Using Antibodies against Asialoglycoprotein Receptor, Carbamoyl Phosphate Synthetase 1 and Pan-Cytokeratin. PLoS ONE 2014, 9, e96185. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, Y.; Xiong, X.; Guo, X.; Zhang, L.; Zhang, X.B.; Zhou, S.B. Codelivery of a pi-pi Stacked Dual Anticancer Drug Combination with Nanocarriers for Overcoming Multidrug Resistance and Tumor Metastasis. Adv. Funct. Mater. 2016, 26, 8266–8280. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Theek, B.; Blenke, E.O.; Pieters, E.H.E.; Fens, M.H.A.M.; Ehling, J.; Schiffelers, R.M.; Storm, G.; van Nostrum, C.F.; et al. Complete Regression of Xenograft Tumors upon Targeted Delivery of Paclitaxel via Pi-Pi Stacking Stabilized Polymeric Micelles. ACS Nano 2015, 9, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, J.; Xu, C.; Shi, L.; Tayier, M.; Zhou, J.; Zhang, J.; Wu, J.; Ye, Z.; Fang, T.; Han, W. Cancer Nanomedicines Stabilized by pi-pi Stacking between Heterodimeric Prodrugs Enable Exceptionally High Drug Loading Capacity and Safer Delivery of Drug Combinations. Theranostics 2017, 7, 3638–3652. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Choi, J.H.; Cho, E.; Lee, J.H.; Yu, S.; Yoon, J.H.; Lee, K.W.; Suh, K.S.; Kim, Y. Integrated analysis of genomic and epigenomic regulation of transcriptome identifies molecular subtypes of liver cancer. J. Hepatol. 2017, 66, S639–S640. [Google Scholar] [CrossRef]
- Zdobnova, T.; Sokolova, E.; Stremovskiy, O.; Karpenko, D.; Telford, W.; Turchin, I.; Balalaeva, I.; Deyev, S. A novel far-red fluorescent xenograft model of ovarian carcinoma for preclinical evaluation of HER2-targeted immunotoxins. Oncotarget 2015, 6, 30919. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Han, J.; Li, D.; Chen, J.; Wang, W.; Xu, W. Synthetic Glycopolypeptide Micelle for Targeted Drug Delivery to Hepatic Carcinoma. Polymers 2018, 10, 611. https://doi.org/10.3390/polym10060611
Li P, Han J, Li D, Chen J, Wang W, Xu W. Synthetic Glycopolypeptide Micelle for Targeted Drug Delivery to Hepatic Carcinoma. Polymers. 2018; 10(6):611. https://doi.org/10.3390/polym10060611
Chicago/Turabian StyleLi, Pengqiang, Jiandong Han, Di Li, Jinjin Chen, Wei Wang, and Weiguo Xu. 2018. "Synthetic Glycopolypeptide Micelle for Targeted Drug Delivery to Hepatic Carcinoma" Polymers 10, no. 6: 611. https://doi.org/10.3390/polym10060611
APA StyleLi, P., Han, J., Li, D., Chen, J., Wang, W., & Xu, W. (2018). Synthetic Glycopolypeptide Micelle for Targeted Drug Delivery to Hepatic Carcinoma. Polymers, 10(6), 611. https://doi.org/10.3390/polym10060611