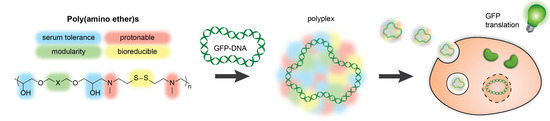
Modular Synthesis of Bioreducible Gene Vectors through Polyaddition of N,N′-Dimethylcystamine and Diglycidyl Ethers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polymer Preparation and Characterization
2.2. Polyplex Formation and Characterization
2.3. Cytotoxicity
2.4. Transfection Efficiency
3. Materials and Methods
3.1. The Synthesis of N,N′-Dimethylcystamine (DMC)
3.2. The Synthesis of N,N′-Bis(2-hydroxyethyl)-N,N′-bis(tert-butoxycarbonyl) ethylenediamine
3.3. The Synthesis of Diglycidyl Ethers
3.4. The Synthesis of Poly(amino ether)s
3.5. Buffer Capacity Titration
3.6. Polyplex Preparation
3.7. Agarose Gel Electrophoresis
3.8. Cytotoxicity
3.9. Transfection Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mingozzi, F.; High, K.A. Therapeutic in Vivo Gene Transfer for Genetic Disease Using Aav: Progress and Challenges. Nat. Rev. Genet. 2011, 12, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.A. State-of-the-Art Gene-Based Therapies: The Road Ahead. Nat. Rev. Genet. 2011, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Seow, Y.; Wood, M.J. Biological Gene Delivery Vehicles: Beyond Viral Vectors. Mol. Ther. 2009, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Chen, C.K.; Ravikrishnan, A.; Rane, S.; Pfeifer, B.A. Overcoming Nonviral Gene Delivery Barriers: Perspective and Future. Mol. Pharm. 2013, 10, 4082–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draghici, B.; Ilies, M.A. Synthetic Nucleic Acid Delivery Systems: Present and Perspectives. J. Med. Chem. 2015, 58, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Non-Viral Gene Delivery Systems. Curr. Opin. Biotechnol. 2002, 13, 128–131. [Google Scholar] [CrossRef]
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the Development of Lipopolyplexes as Efficient Non-Viral Gene Delivery Systems. J. Control. Release 2016, 236, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Zhang, S.; Kos, P.; Xiong, H.; Zhou, K.; Perelman, S.S.; Zhu, H.; Siegwart, D.J. Non-Viral Crispr/Cas Gene Editing in Vitro and in Vivo Enabled by Synthetic Nanoparticle Co-Delivery of Cas9 Mrna and Sgrna. Angew. Chem. Int. Ed. Engl. 2017, 56, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Li, M.; Lee, C.M.; Chakraborty, S.; Kim, H.W.; Bao, G.; Leong, K.W. Crispr/Cas9-Based Genome Editing for Disease Modeling and Therapy: Challenges and Opportunities for Nonviral Delivery. Chem. Rev. 2017, 117, 9874–9906. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Song, C.Q.; Dorkin, J.R.; Zhu, L.J.; Li, Y.; Wu, Q.; Park, A.; Yang, J.; Suresh, S.; Bizhanova, A.; et al. Therapeutic Genome Editing by Combined Viral and Non-Viral Delivery of Crispr System Components in Vivo. Nat. Biotechnol. 2016, 34, 328–333. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.; Khalil, I.; Ali, I.; Yacoub, M. Updates on Smart Polymeric Carrier Systems for Protein Delivery. Drug Dev. Ind. Pharm. 2017, 43, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Yuk, S.H. Polymeric Protein Delivery Systems. Prog. Polym. Sci. 2007, 32, 669–697. [Google Scholar] [CrossRef]
- Kataoka, K.; Harashima, H. Gene Delivery Systems: Viral Vs. Non-Viral Vectors. Adv. Drug Deliv. Rev. 2001, 52, 151. [Google Scholar] [CrossRef]
- Miyata, K.; Nishiyama, N.; Kataoka, K. Rational Design of Smart Supramolecular Assemblies for Gene Delivery: Chemical Challenges in the Creation of Artificial Viruses. Chem. Soc. Rev. 2012, 41, 2562–2574. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Narain, R. Progress of Raft Based Polymers in Gene Delivery. Prog. Polym. Sci. 2013, 38, 767–790. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and Development of Polymers for Gene Delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.S.; Schellinger, J.G.; Shi, J.; Convertine, A.J.; Stayton, P.S.; Pun, S.H. Application of Living Free Radical Polymerization for Nucleic Acid Delivery. Acc. Chem. Res. 2012, 45, 1089–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, R.; Tang, L.; Ma, L.; Tu, C.; Baumgartner, R.; Cheng, J. Smart Chemistry in Polymeric Nanomedicine. Chem. Soc. Rev. 2014, 43, 6982–7012. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Facing the Truth About Nanotechnology in Drug Delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Jain, R.K. Strategies for Advancing Cancer Nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, V.A. DNA Condensation by Multivalent Cations. Biopolymers 1997, 44, 269–282. [Google Scholar] [CrossRef]
- Perevyazko, I.Y.; Bauer, M.; Pavlov, G.M.; Hoeppener, S.; Schubert, S.; Fischer, D.; Schubert, U.S. Polyelectrolyte Complexes of DNA and Linear PEI: Formation, Composition and Properties. Langmuir 2012, 28, 16167–16176. [Google Scholar] [CrossRef] [PubMed]
- Meneksedag-Erol, D.; Tang, T.; Uludag, H. Molecular Modeling of Polynucleotide Complexes. Biomaterials 2014, 35, 7068–7076. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, P.; Schlenoff, J.B. Saloplastics: Processing Compact Polyelectrolyte Complexes. Adv. Mater. 2015, 27, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Vianello, F.; Magliulo, G.; Thomas, T.; Thomas, T.J. Nanoparticle Strategies for Cancer Therapeutics: Nucleic Acids, Polyamines, Bovine Serum Amine Oxidase and Iron Oxide Nanoparticles (Review). Int. J. Oncol. 2015, 46, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Tajmir-Riahi, H.A.; Thomas, T. Polyamine-DNA Interactions and Development of Gene Delivery Vehicles. Amino Acids 2016, 48, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Thomas, T. Collapse of DNA in Packaging and Cellular Transport. Int. J. Biol. MacroMol. 2018, 109, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Midoux, P.; Breuzard, G.; Gomez, J.P.; Pichon, C. Polymer-Based Gene Delivery: A Current Review on the Uptake and Intracellular Trafficking of Polyplexes. Curr. Gene Ther. 2008, 8, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualch, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in-Vivo-Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.-P. The Proton Sponge: A Trick to Enter Cells the Viruses Did Not Exploit. CHIMIA Int. J. Chem. 1997, 51, 34–36. [Google Scholar]
- Liang, W.; Lam, J.K.W. Endosomal escape pathways for non-viral nucleic acid delivery systems. In Molecular Regulation of Endocytosis; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Sonawane, N.D.; Szoka, F.C., Jr.; Verkman, A.S. Chloride Accumulation and Swelling in Endosomes Enhances DNA Transfer by Polyamine-DNA Polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knorr, V.; Russ, V.; Allmendinger, L.; Ogris, M.; Wagner, E. Acetal Linked Oligoethylenimines for Use as Ph-Sensitive Gene Carriers. Bioconjug. Chem. 2008, 19, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, M.; Meng, F.; Zhong, Z. Non-Viral Gene Transfection in Vitro Using Endosomal Ph-Sensitive Reversibly Hydrophobilized Polyethylenimine. Biomaterials 2011, 32, 9109–9119. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.M.; Anderson, D.G.; Putnam, D.; Langer, R. Accelerated Discovery of Synthetic Transfection Vectors: Parallel Synthesis and Screening of a Degradable Polymer Library. J. Am. Chem. Soc. 2001, 123, 8155–8156. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.M.; Langer, R. Degradable Poly(Beta-Amino Esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Law, B.; Tung, C.H. Proteolysis: A Biological Process Adapted in Drug Delivery, Therapy, and Imaging. Bioconj. Chem. 2009, 20, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Kate, P.; Torchilin, V.P. Matrix Metalloprotease 2-Responsive Multifunctional Liposomal Nanocarrier for Enhanced Tumor Targeting. ACS Nano 2012, 6, 3491–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Wang, T.; Perche, F.; Taigind, A.; Torchilin, V.P. Enhanced Anticancer Activity of Nanopreparation Containing an Mmp2-Sensitive Peg-Drug Conjugate and Cell-Penetrating Moiety. Proc. Natl. Acad. Sci. USA 2013, 110, 17047–17052. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V.P. Matrix Metalloproteinase 2-Sensitive Multifunctional Polymeric Micelles for Tumor-Specific Co-Delivery of siRNA and Hydrophobic Drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.; Huck, W.T.; Genzer, J.; Muller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Bauhuber, S.; Hozsa, C.; Breunig, M.; Gopferich, A. Delivery of Nucleic Acids Via Disulfide-Based Carrier Systems. Adv. Mater. 2009, 21, 3286–3306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-Responsive Nano-Vehicles as a Promising Platform for Targeted Intracellular Drug and Gene Delivery. J. Control. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-Cleavage-Triggered Chemosensors and Their Biological Applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef] [PubMed]
- Oupicky, D.; Li, J. Bioreducible Polycations in Nucleic Acid Delivery: Past, Present, and Future Trends. MacroMol. Biosci. 2014, 14, 908–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Engbersen, J.F. The Role of the Disulfide Group in Disulfide-Based Polymeric Gene Carriers. Expert Opin. Drug Deliv. 2009, 6, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Elzes, M.R.; Akeroyd, N.; Engbersen, J.F.; Paulusse, J.M. Disulfide-Functional Poly(Amido Amine)S with Tunable Degradability for Gene Delivery. J. Control. Release 2016, 244, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, M.A.; Guo, W.J.; Lee, R.J. Efficient Gene Transfer Using Reversibly Cross-Linked Low Molecular Weight Polyethylenimine. Bioconj. Chem. 2001, 12, 989–994. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Shen, J. The Development and Characterization of a Glutathione-Sensitive Cross-Linked Polyethylenimine Gene Vector. Biomaterials 2006, 27, 5292–5298. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, R.C.; Etrych, T.; Briggs, S.S.; Preece, J.A.; Ulbrich, K.; Seymour, L.W. Polymer-Coated Polyethylenimine/DNA Complexes Designed for Triggered Activation by Intracellular Reduction. J. Gene Med. 2004, 6, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Mo, H.; Koo, H.; Park, J.Y.; Cho, M.Y.; Jin, G.W.; Park, J.S. Visualization of the Degradation of a Disulfide Polymer, Linear Poly(Ethylenimine Sulfide), for Gene Delivery. Bioconj. Chem. 2007, 18, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhong, Z.; Zhuo, R. Disulfide Cross-Linked Polyethylenimines (PEI) Prepared Via Thiolation of Low Molecular Weight PEI as Highly Efficient Gene Vectors. Bioconj. Chem. 2008, 19, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, Y.; Harada, A.; Kataoka, K. Environment-Sensitive Stabilization of Core-Shell Structured Polyion Complex Micelle by Reversible Cross-Linking of the Core through Disulfide Bond. J. Am. Chem. Soc. 1999, 121, 11247–11248. [Google Scholar] [CrossRef]
- Miyata, K.; Kakizawa, Y.; Nishiyama, N.; Harada, A.; Yamasaki, Y.; Koyama, H.; Kataoka, K. Block Catiomer Polyplexes with Regulated Densities of Charge and Disulfide Cross-Linking Directed to Enhance Gene Expression. J. Am. Chem. Soc. 2004, 126, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, D.L.; Smiley, E.; Kwok, K.Y.; Rice, K.G. Low Molecular Weight Disulfide Cross-Linking Peptides as Nonviral Gene Delivery Carriers. Bioconj. Chem. 2000, 11, 901–909. [Google Scholar] [CrossRef]
- McKenzie, D.L.; Kwok, K.Y.; Rice, K.G. A Potent New Class of Reductively Activated Peptide Gene Delivery Agents. J. Biol. Chem. 2000, 275, 9970–9977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, H.; Yang, W.; Cheng, Y. Disulfide Cross-Linked Low Generation Dendrimers with High Gene Transfection Efficacy, Low Cytotoxicity, and Low Cost. J. Am. Chem. Soc. 2012, 134, 17680–17687. [Google Scholar] [CrossRef] [PubMed]
- Kozielski, K.L.; Tzeng, S.Y.; Green, J.J. A Bioreducible Linear Poly(Beta-Amino Ester) for siRNA Delivery. Chem. Commun. 2013, 49, 5319–5321. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhong, Z.; Lok, M.C.; Jiang, X.; Hennink, W.E.; Feijen, J.; Engbersen, J.F. Novel Bioreducible Poly(Amido Amine)S for Highly Efficient Gene Delivery. Bioconj. Chem. 2007, 18, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yan, J.; You, Y. Synthesis of Bioreducible and Acid Labile Poly(Amido Amine)S Via Michael-Addition Reactions and Their Application in Gene Delivery. J. Control. Release 2011, 152 (Suppl. 1), e179–e181. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Xu, R.; Kim, S.H.; Bull, D.A.; Kim, S.W. A Family of Bioreducible Poly(Disulfide Amine)S for Gene Delivery. Biomaterials 2009, 30, 5804–5814. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P. Poly(Amidoamine)S: Past, Present, and Perspectives. J. Polym. Sci. Polym. Chem. 2013, 51, 2319–2353. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Langer, R. Translating Materials Design to the Clinic. Nat. Mater. 2013, 12, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Akita, H.; Harashima, H. A Multifunctional Envelope Type Nano Device (Mend) for Gene Delivery to Tumours Based on the Epr Effect: A Strategy for Overcoming the Peg Dilemma. Adv. Drug Deliv. Rev. 2011, 63, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.; Ramos, J.; Potta, T.; Rege, K. Generation of a Focused Poly(Amino Ether) Library: Polymer-Mediated Transgene Delivery and Gold-Nanorod Based Theranostic Systems. Theranostics 2012, 2, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Ferruti, P.; Marchisio, M.A.; Duncan, R. Poly(Amido-Amine)S: Biomedical Applications. MacroMol. Rapid Commun. 2002, 23, 332–355. [Google Scholar] [CrossRef]
- Emilitri, E.; Ferruti, P.; Annunziata, R.; Ranucci, E.; Rossi, M.; Falciola, L.; Mussini, P.; Chiellini, F.; Bartoli, C. Novel Amphoteric Cystine-Based Poly(Amidoamine)S Responsive to Redox Stimuli. Macromolecules 2007, 40, 4785–4793. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Green, J.J.; Chan, J.M.; Longer, R.; Anderson, D.G. Polymeric Materials for Gene Delivery and DNA Vaccination. Adv. Mater. 2009, 21, 847–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niebel, Y.; Buschmann, M.D.; Lavertu, M.; De Crescenzo, G. Combined Analysis of Polycation/Odn Polyplexes by Analytical Ultracentrifugation and Dynamic Light Scattering Reveals Their Size, Refractive Index Increment, Stoichiometry, Porosity, and Molecular Weight. Biomacromolecules 2014, 15, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, R.C.; Yi, W.J.; Zhao, Z.G. Biodegradable Poly(Amino Ester) with Aromatic Backbone as Efficient Nonviral Gene Delivery Vectors. Molecules 2017, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jung, S.; Si, G.; Cheng, R.; Meng, F.; Zhu, X.; Park, T.G.; Zhong, Z. Cationic Methacrylate Copolymers Containing Primary and Tertiary Amino Side Groups: Controlled Synthesis Via Raft Polymerization, DNA Condensation, and in Vitro Gene Transfection. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2869–2877. [Google Scholar] [CrossRef]
- Luo, X.H.; Huang, F.W.; Qin, S.Y.; Wang, H.F.; Feng, J.; Zhang, X.Z.; Zhuo, R.X. A Strategy to Improve Serum-Tolerant Transfection Activity of Polycation Vectors by Surface Hydroxylation. Biomaterials 2011, 32, 9925–9939. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, F.; Yuan, Z.F.; Zhuo, R.X. Influence of Hydroxyl Groups on the Biological Properties of Cationic Polymethacrylates as Gene Vectors. Acta Biomater. 2010, 6, 2658–2665. [Google Scholar] [CrossRef] [PubMed]
- Piest, M.; Lin, C.; Mateos-Timoneda, M.A.; Lok, M.C.; Hennink, W.E.; Feijen, J.; Engbersen, J.F. Novel Poly(Amido Amine)S with Bioreducible Disulfide Linkages in Their Diamino-Units: Structure Effects and in Vitro Gene Transfer Properties. J. Control. Release 2008, 130, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Feijen, J.; Lok, M.C.; Hennink, W.E.; Christensen, L.V.; Yockman, J.W.; Kim, Y.H.; Kim, S.W. Low Molecular Weight Linear Polyethylenimine-B-Poly(Ethylene Glycol)-B-Polyethylenimine Triblock Copolymers: Synthesis, Characterization, and in Vitro Gene Transfer Properties. Biomacromolecules 2005, 6, 3440–3448. [Google Scholar] [CrossRef] [PubMed]






| Poly(amino ether) | Yield a (%) | Mwb (kDa) | PDI b | Degree of Polymerization b | Buffer Capacity c (%) |
|---|---|---|---|---|---|
| DMC1 | 31.6 | 3.3 | 1.6 | 4.6 | 36.0 |
| DMC2 | 33.2 | 4.0 | 2.0 | 4.4 | 50.4 |
| DMC3 | 16.9 | 2.5 | 1.5 | 3.8 | 64.0 |
| DMC4 | 13.9 | 2.9 | 1.7 | 4.1 | 34.4 |
| DMC5 | 43.7 | 3.8 | 1.8 | 5.0 | 53.6 |
| DMC6 | 40.1 | 4.2 | 1.8 | 6.1 | 36.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, G.; Elzes, M.R.; Engbersen, J.F.J.; Paulusse, J.M.J. Modular Synthesis of Bioreducible Gene Vectors through Polyaddition of N,N′-Dimethylcystamine and Diglycidyl Ethers. Polymers 2018, 10, 687. https://doi.org/10.3390/polym10060687
Si G, Elzes MR, Engbersen JFJ, Paulusse JMJ. Modular Synthesis of Bioreducible Gene Vectors through Polyaddition of N,N′-Dimethylcystamine and Diglycidyl Ethers. Polymers. 2018; 10(6):687. https://doi.org/10.3390/polym10060687
Chicago/Turabian StyleSi, Guoying, M. Rachèl Elzes, Johan F. J. Engbersen, and Jos M. J. Paulusse. 2018. "Modular Synthesis of Bioreducible Gene Vectors through Polyaddition of N,N′-Dimethylcystamine and Diglycidyl Ethers" Polymers 10, no. 6: 687. https://doi.org/10.3390/polym10060687
APA StyleSi, G., Elzes, M. R., Engbersen, J. F. J., & Paulusse, J. M. J. (2018). Modular Synthesis of Bioreducible Gene Vectors through Polyaddition of N,N′-Dimethylcystamine and Diglycidyl Ethers. Polymers, 10(6), 687. https://doi.org/10.3390/polym10060687