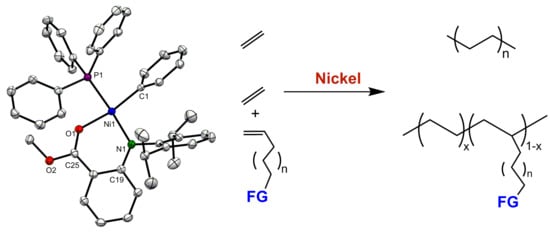
Ethylene Polymerization and Copolymerization with Polar Monomers Using Nickel Complexes Bearing Anilinobenzoic Acid Methyl Ester Ligand
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Analytical Procedure
2.3. General Polymerization Procedure
2.4. Synthesis of Ligands and Complexes
2.4.1. Synthesis of 2-Anilinobenzoic Acid Methyl Ester Ligand L1
2.4.2. Synthesis of 2-(2,6-Diisopropylaniline)-Methyl Benzoate Ligand L2
2.4.3. Synthesis of Nickel Complex C1
2.4.4. Synthesis of Nickel Complex C2
3. Results and Discussion
3.1. Synthesis and Molecular Structure of Nickel Complexes
3.2. Ethylene Polymerization
3.3. Copolymerization of Ethylene with Polar Monomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sturzel, M.; Mihan, S.; Mulhaupt, R. From Multisite Polymerization Catalysis to Sustainable Materials and All-Polyolefin Composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Franssen, N.M.G.; Reek, J.N.H.; Bruin, B.D. Synthesis of Functional ‘Polyolefins’: State of the Art and Remaining Challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef] [PubMed]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.X. Coordination Polymerization of Polar Vinyl Monomers by Single-Site Metal Catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.H.; Guan, Z. Designing late-transition metal catalysts for olefin insertion polymerization and copolymerization. Chem. Commun. 2010, 46, 7879–7893. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ye, Z. Hyperbranched polyethylenes by chain walking polymerization: Synthesis, properties, functionalization, and applications. Polym. Chem. 2012, 3, 286–301. [Google Scholar] [CrossRef]
- Nakamura, A.; Anselment, T.M.J.; Claverie, J.; Goodall, B.; Jordan, R.F.; Mecking, S.; Rieger, B.; Sen, A.; van Leeuwen, P.W.N.M.; Nozaki, K. Ortho-Phosphinobenzenesulfonate: A Superb Ligand for Palladium-Catalyzed Coordination-Insertion Copolymerization of Polar Vinyl Monomers. Acc. Chem. Res. 2013, 46, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xu, L.; Dong, Z.; Peng, X. Designing polyethylenes of complex chain architectures via Pd–diimine-catalyzed “living” ethylene. Chem. Commun. 2013, 49, 6235–6255. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.L.; Pan, L.; Song, D.P.; Li, Y.S. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Carrow, B.P.; Nozaki, K. Transition-Metal-Catalyzed Functional Polyolefin Synthesis: Effecting Control through Chelating Ancillary Ligand Design and Mechanistic Insights. Macromolecules 2014, 47, 2541–2555. [Google Scholar] [CrossRef]
- Long, B.K.; Eagan, J.M.; Mulzer, M.; Coates, G.W. SemiCrystalline Polar Polyethylene: Ester-Functionalized Linear Polyolefins Enabled by a Functional-Group-Tolerant, Cationic Nickel Catalyst. Angew. Chem. Int. Ed. 2016, 55, 7106–7110. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Nakano, R.; Ito, S.; Nozaki, K. Copolymerization of Ethylene and Polar Monomers by Using Ni/IzQO Catalysts. Angew. Chem. Int. Ed. 2016, 55, 2835–2839. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.S.; Sato, N.; Tanna, A.; Oishi, Y.; Konishi, Y.; Shimizu, F. Nickel Catalyzed Copolymerization of Ethylene and Alkyl Acrylates. J. Am. Chem. Soc. 2017, 139, 3611–3614. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, C.L. Design of High-Performance Phosphine Sulfonate Nickel Catalysts for Ethylene Polymerization and Copolymerization with Polar Monomers. ACS Catal. 2017, 7, 1308–1312. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, L.J.; Tanaka, R.; Shiono, T.; Cai, Z. Highly Robust Nickel Catalysts Containing Anilinonaphthoquinone Ligand for Copolymerization of Ethylene and Polar Monomers. Macromolecules 2017, 50, 9216–9221. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.B.; Luo, Y.; Chen, C.L. A Second-Coordination-Sphere Strategy to Modulate Nickel- and Palladium-Catalyzed Olefin Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 11604–11609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, C.L. Influence of Polyethylene Glycol Unit on Palladium- and NickelCatalyzed Ethylene Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 14672–14676. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Tao, W.J.; Yasuda, H.; Ito, S.; Oishi, Y.; Ohtaki, H.; Tanna, A.; Tayano, T.; Nozaki, K. Nickel-Catalyzed Propylene/Polar Monomer Copolymerization. ACS Macro Lett. 2018, 7, 213–217. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.L. A Versatile Ligand Platform for Palladium- and Nickel-Catalyzed Ethylene Copolymerization with Polar Monomers. Angew. Chem. Int. Ed. 2018, 57, 3094–3098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mesgar, M.; White, P.S.; Daugulis, O.; Brookhart, M. Synthesis of Branched Ultrahigh-Molecular-Weight Polyethylene Using Highly Active Neutral, Single-Component Ni(II) Catalysts. ACS Catal. 2015, 5, 631–636. [Google Scholar] [CrossRef]
- Keim, W.; Kowaldt, F.H.; Goddard, R.; Kruger, C. Novel coordination of (benzoylmethylene) triphenylphosphorane in a nickel oligomerization catalyst. Angew. Chem. Int. Ed. 1978, 17, 466–467. [Google Scholar] [CrossRef]
- Klabunde, U.; Ittel, S.D. Nickel catalysis for ethylene homo- and co-polymerization. J. Mol. Catal. 1987, 41, 123–134. [Google Scholar] [CrossRef]
- Starzewski, K.A.O.; Witte, J. Highly Active Ylide-Nickel Catalysts for the Polymerization of Ethylene. Angew. Chem. Int. Ed. 1985, 24, 599–601. [Google Scholar] [CrossRef]
- Wang, C.; Friedrich, S.; Younkin, T.R.; Li, R.T.; Grubbs, R.H.; Bansleben, D.A.; Day, M.W. Neutral Nickel(II)-Based Catalysts for Ethylene Polymerization. Organometallics 1998, 17, 3149–3151. [Google Scholar] [CrossRef] [Green Version]
- Younkin, T.R.; Connor, E.F.; Henderson, J.I.; Friedrich, S.K.; Grubbs, R.H.; Bansleben, D.A. Neutral, Single-Component Nickel (II) Polyolefin Catalysts That Tolerate Heteroatoms. Science 2000, 287, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.F.; Younkin, T.R.; Henderson, J.I.; Waltman, A.W.; Grubbs, R.H. Synthesis of neutral nickel catalysts for ethylene polymerization–the influence of ligand size on catalyst stability. Chem. Commun. 2003, 18, 2272–2273. [Google Scholar] [CrossRef]
- Waltman, A.W.; Younkin, T.R.; Grubbs, R.H. Insights into the deactivation of neutral nickel ethylene polymerization catalysts in the presence of functionalized olefins. Organometallics 2004, 23, 5121–5123. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, G.X.; Weng, L.H.; Wang, F. Synthesis, Molecular Structures, and Norbornene Addition Polymerization Activity of the Neutral Nickel Catalysts Supported by β-Diketiminato [N, N], Ketiminato [N, O], and Schiff-Base [N, O] Ligands. Organometallics 2004, 23, 3270–3275. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wu, G.; Bazan, G.C. Remote Activation of Nickel Catalysts for Ethylene Oligomerization. Angew. Chem. Int. Ed. 2005, 4, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Li, Y.G.; Li, Y.S.; Chen, Y.X.; Hu, N.H. Copolymerization of Ethylene with Methyl Methacrylate with Neutral Nickel(II) Complexes Bearing β-Ketoiminato Chelate Ligands. Organometallics 2005, 24, 2502–2510. [Google Scholar] [CrossRef]
- Zhang, L.; Brookhart, M.; White, P.S. Synthesis, Characterization, and Ethylene Polymerization Activities of Neutral Nickel(II) Complexes Derived from Anilino-Substituted Enone Ligands Bearing Trifluoromethyl and Trifluoroacetyl Substituents. Organometallics 2006, 25, 1868–1874. [Google Scholar] [CrossRef]
- Yu, S.M.; Berkefeld, A.; Gottker-Schnetmann, I.; Muller, G.; Mecking, S. Synthesis of Aqueous Polyethylene Dispersions with Electron-Deficient Neutral Nickel(II) Catalysts with Enolatoimine Ligands. Macromolecules 2007, 40, 421–428. [Google Scholar] [CrossRef]
- Song, D.P.; Ye, W.P.; Wang, Y.X.; Liu, J.Y.; Li, Y.S. Highly Active Neutral Nickel(II) Catalysts for Ethylene Polymerization Bearing Modified, β-Ketoiminato Ligands. Organometallics 2009, 28, 5697–5704. [Google Scholar] [CrossRef]
- Song, D.P.; Wu, J.Q.; Ye, W.P.; Mu, H.L.; Li, Y.S. Accessible, Highly Active Single-Component β-Ketiminato Neutral Nickel(II) Catalysts for Ethylene Polymerization. Organometallics 2010, 29, 2306–2314. [Google Scholar] [CrossRef]
- Song, D.P.; Wang, Y.X.; Mu, H.L.; Li, B.X.; Li, Y.S. Observations and Mechanistic Insights on Unusual Stability of Neutra Nickel Complexes with a Sterically Crowded Metal Center. Organometallics 2011, 30, 925–934. [Google Scholar] [CrossRef]
- Song, D.P.; Shi, X.C.; Wang, Y.X.; Yang, J.X.; Li, Y.S. Ligand Steric and Electronic Effects on β-Ketiminato Neutral Nickel(II) Olefin Polymerization Catalysts. Organometallics 2012, 31, 966–975. [Google Scholar] [CrossRef]
- Hicks, F.A.; Brookhart, M. A Highly Active Anilinotropone-Based Neutral Nickel(II) Catalyst for Ethylene Polymerization. Organometallics 2001, 20, 3217–3219. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Brookhart, M. A Highly Active Anilinoperinaphthenone-Based Neutral Nickel(II) Catalyst for Ethylene Polymerization. Organometallics 2003, 22, 250–256. [Google Scholar] [CrossRef]
- Hicks, F.A.; Jenkins, C.J.; Brookhart, M. Synthesis and Ethylene Polymerization Activity of a Series of 2-Anilinotropone-Based Neutral Nickel(II) Catalysts. Organometallics 2003, 22, 3533–3545. [Google Scholar] [CrossRef]
- Jenkins, J.C.; Brookhart, M. A Mechanistic Investigation of the Polymerization of Ethylene Catalyzed by Neutral Ni(II) Complexes Derived from Bulky Anilinotropone Ligands. J. Am. Chem. Soc. 2004, 126, 5827–5842. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Nakayama, Y.; Ikeda, T.; Shiono, T. Synthesis of Uniquely Branched Polyethylene by Anilinonaphthoquinone Ligated Nickel Complex Activated with Tris(pentafluorophenyl)borane. Macromol. Rapid Commun. 2006, 27, 1418–1423. [Google Scholar] [CrossRef]
- Okada, M.; Nakayama, Y.; Shiono, T. Synthesis of anilinonaphthoquinone-based nickel complexes and their application for olefin polymerization. J. Organomet. Chem. 2007, 692, 5183–5189. [Google Scholar] [CrossRef]
- Okada, M.; Nakayama, Y.; Shiono, T. Heterogenization of an Anilinonaphthoquinone-Chelated Nickel Complex for Ethylene Polymerization Using Silica-Supported Modified Methylaluminoxane. Mcromol. Chem. Phys. 2014, 215, 1792–1796. [Google Scholar] [CrossRef]
- Okada, M.; Nakayama, Y.; Shiono, T. Highly Soluble Polynorbornene Prepared by an Anilinonaphthoquinone-Ligated Nickel Complex via Coordination-Insertion Polymerization. J. Organomet. Chem. 2015, 798, 384–387. [Google Scholar] [CrossRef]
- Cheng, H.L.; Cai, Z. (Anilino)anthraquinone Nickel-Catalyzed Random Copolymerization of Norbornene and Ethylene. ChemCatChem 2018, 10, 497–500. [Google Scholar] [CrossRef]
- Cai, Z.; Donghua University, Shanghai, China. Unpublished results of our group. 2018.
- Beletskaya, I.P.; Bessmertnykh, A.G.; Averin, A.D.; Denat, F.; Guilard, R. Palladium-Catalyzed Amination of 1,8- and 1,5-Dichloroanthracenes and 1,8- and 1,5-Dichloroanthraquinones. Eur. J. Org. Chem. 2005, 36, 281–305. [Google Scholar] [CrossRef]
- Zeller, A.; Herdtweck, E.; Strassner, T. Structural Characterization and a New One-Pot Synthesis of trans-Chloro(phenyl)bis(triphenylphosphane)nickel(II). Eur. J. Inorg. Chem. 2003, 2003, 1802–1806. [Google Scholar] [CrossRef]
- Chandran, D.; Bae, C.; Ahn, I.; Ha, C.S.; Kim, I. Neutral Ni(II) Complexes Based on Keto-Enamine Salicylideneanilines Active for Selective Dimerization of Ethylene. J. Organomet. Chem. 2009, 694, 1254–1258. [Google Scholar] [CrossRef]
- Wu, J.L.; Pan, Q.M.; Rempel, G.L. Solubility of Ethylene in Toluene and Toluene/Styrene–Butadiene Rubber Solutions. J. Appl. Polym. Sci. 2005, 96, 645–649. [Google Scholar] [CrossRef]
- Terao, H.; Ishii, S.; Mitani, M.; Tanaka, H.; Fujita, T. Ethylene/Polar Monomer Copolymerization Behavior of Bis(phenoxy–imine)Ti Complexes: Formation of Polar Monomer Copolymers. J. Am. Chem. Soc. 2008, 130, 17636–17637. [Google Scholar] [CrossRef] [PubMed]





| Entry | Catalyst | Cocatalyst | P (MPa) | Temp (°C) | Yield (g) | Activity (kg·mol−1·h−1) | Mnb (103) | PDI b | Tmc (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C2 | - | 1.5 | 40 | 0.06 | 36 | 10.4 | 2.14 | 97.0 |
| 2 d | C2 | dMMAO | 1.5 | 40 | oligomer | 616 | - | - | - |
| 3 | C1 | Ni(COD)2 | 1.5 | 40 | trace | - | - | - | - |
| 4 | C2 | Ni(COD)2 | 1.5 | 40 | 1.29 | 774 | 12.2 | 2.08 | 90. 6 |
| 5 | C2 | Ni(COD)2 | 1.5 | 20 | 0.14 | 84 | 76.3 | 2.28 | 125.9 |
| 6 | C2 | Ni(COD)2 | 1.5 | 60 | 0.56 | 336 | 6.9 | 2.11 | 45.2 |
| 7 | C2 | Ni(COD)2 | 0.5 | 40 | 0.17 | 99 | 8.6 | 1.85 | 87.3 |
| 8 | C2 | Ni(COD)2 | 3.0 | 40 | 1.46 | 876 | 19.1 | 2.54 | 95.2 |
| Entry | Comonomer (mmol) | Cocatalyst | Yield (g) | Activity (kg·mol−1·h−1) | Mnb (104) | PDI b | Tmc (°C) | Incorp. d (mol %) |
|---|---|---|---|---|---|---|---|---|
| 1 | HAc(30) | Ni(COD)2 | 0.05 | 5 | 0.87 | 1.92 | 97.8 | 1.01 |
| 2 | HAc(10) | dMMAO | 2.77 | 277 | 1.97 | 2.00 | 97.9 | 0.35 |
| 3 | HAc(30) | dMMAO | 2.01 | 201 | 1.31 | 2.39 | 78.1 | 0.88 |
| 4 | HAc(60) | dMMAO | 1.74 | 174 | 1.17 | 2.16 | 71.4 | 1.33 |
| 5 | NBAc (30) | dMMAO | 1.14 | 114 | 1.52 | 3.21 | 67.1 | 3.20 |
| 6 | VAc(30) | dMMAO | trace | - | - | - | - | - |
| 7 | MA(30) | dMMAO | trace | - | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Su, Y.; Hu, Y.; Zhang, X.; Cai, Z. Ethylene Polymerization and Copolymerization with Polar Monomers Using Nickel Complexes Bearing Anilinobenzoic Acid Methyl Ester Ligand. Polymers 2018, 10, 754. https://doi.org/10.3390/polym10070754
Cheng H, Su Y, Hu Y, Zhang X, Cai Z. Ethylene Polymerization and Copolymerization with Polar Monomers Using Nickel Complexes Bearing Anilinobenzoic Acid Methyl Ester Ligand. Polymers. 2018; 10(7):754. https://doi.org/10.3390/polym10070754
Chicago/Turabian StyleCheng, Hailong, Yue Su, Yanming Hu, Xuequan Zhang, and Zhengguo Cai. 2018. "Ethylene Polymerization and Copolymerization with Polar Monomers Using Nickel Complexes Bearing Anilinobenzoic Acid Methyl Ester Ligand" Polymers 10, no. 7: 754. https://doi.org/10.3390/polym10070754
APA StyleCheng, H., Su, Y., Hu, Y., Zhang, X., & Cai, Z. (2018). Ethylene Polymerization and Copolymerization with Polar Monomers Using Nickel Complexes Bearing Anilinobenzoic Acid Methyl Ester Ligand. Polymers, 10(7), 754. https://doi.org/10.3390/polym10070754