Fabrication of Reactive Poly(Phenyl-Substituted Siloxanes/Silsesquioxanes) with Si‒H and Alkoxy Functional Groups via the Piers–Rubinsztajn Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dehydrocarbon Polycondensation of Diphenyldimethoxysilane (DPDMS) with Hydrogen-Containing Siloxanes (HCS)
2.3. Dehydrocarbon Polycondensation of Diphenyldiethoxysilane (DPDES) with HCS
2.4. Measurements
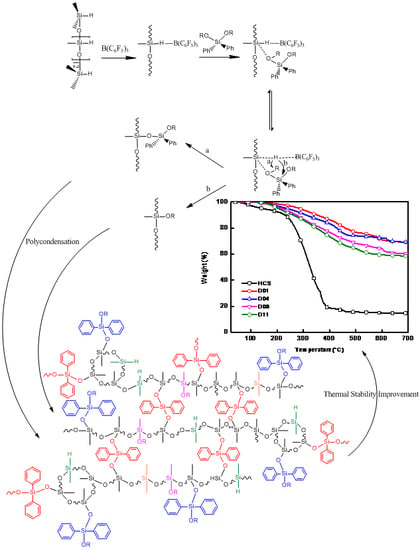
3. Results and Discussion
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Massey, A.G.; Park, A.J. Perfluorophenyl derivatives of the elements: I. Tris(pentafluorophenyl)boron. J. Organomet. Chem. 1964, 2, 245–250. [Google Scholar] [CrossRef]
- Massey, A.G.; Park, A.J. Perfluorophenyl derivatives of the elements: VII. Further studies on tris(pentafluorophenyl)boron. J. Organomet. Chem. 1966, 5, 218–225. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cation-like homogeneous olefin polymerization catalysts based upon zirconocene alkyls and tris(pentafluorophenyl)borane. J. Am. Chem. Soc. 1991, 113, 3623–3625. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic zirconocene olefin polymerization catalysts based on the organo-lewis acid tris(pentafluorophenyl)borane. A synthetic, structural, solution dynamic, and polymerization catalytic study. J. Am. Chem. Soc. 1994, 116, 10015–10031. [Google Scholar] [CrossRef]
- Parks, D.J.; Piers, W.E. Cheminform abstract: Tris(pentafluorophenyl)boron-catalyzed hydrosilation of aromatic aldehydes, ketones, and esters. J. Cheminf. 1997, 28, 9440–9441. [Google Scholar] [CrossRef]
- Parks, D.J.; Blackwell, J.M.; Piers, W.E. Studies on the mechanism of b(c(6)f(5))(3)-catalyzed hydrosilation of carbonyl functions. J. Org. Chem. 2000, 65, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Piers, W.E. The chemistry of perfluoroaryl boranes. Adv. Organomet. Chem. 2004, 52, 1–76. [Google Scholar]
- Berkefeld, A.; Piers, W.E.; Parvez, M. Tandem frustrated lewis pair/tris(pentafluorophenyl)borane-catalyzed deoxygenative hydrosilylation of carbon dioxide. J. Am. Chem. Soc. 2010, 132, 10660–10661. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.A.; Grande, J.B.; Ganachaud, F. New synthetic strategies for structured silicones using B(C6F5)3. Adv. Polym. Sci. 2010, 235, 161–183. [Google Scholar]
- Cheesman, B.T.; Gates, P.J.; Castle, T.C.; Cosgrove, T.; Prescott, S.W. Linear and star architecture methacrylate-functionalised pdms. Mater. Today Commun. 2015, 3, 122–129. [Google Scholar] [CrossRef]
- Szawiola, A.M.; de Melo Souza, N.; Lessard, B.H.; Bender, T.P. Phenoxylated siloxane-based polymers via the piers-rubinsztajn process. Polym. Int. 2017, 66, 1324–1328. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liang, S.; Yu, L.Y.; Skov, A.L.; Etmimi, H.M.; Mallon, P.E.; Adronov, A.; Brook, M.A. Silicone-modified graphene oxide fillers via the piers-rubinsztajn reaction. J. Polym. Sci. Pol. Chem. 2016, 54, 2379–2385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.F.; Chen, Y.; Brook, M.A. Reductive degradation of lignin and model compounds by hydrosilanes. ACS Sustain. Chem. Eng. 2014, 2, 1983–1991. [Google Scholar] [CrossRef]
- Macphail, B.; Brook, M.A. Controlling silicone-saccharide interfaces: Greening silicones. Green Chem. 2017, 19, 4373–4379. [Google Scholar] [CrossRef]
- Madsen, F.B.; Javakhishvili, I.; Jensen, R.E.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. Synthesis of telechelic vinyl/allyl functional siloxane copolymers with structural control. Polym. Chem. 2014, 5, 7054–7061. [Google Scholar] [CrossRef] [Green Version]
- Homrighausen, C.L.; Keller, T.M. Synthesis of hydroxy-terminated, oligomeric poly(silarylene disiloxane)s via rhodium-catalyzed dehydrogenative coupling and their use in the aminosilane–disilanol polymerization reaction. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1334–1341. [Google Scholar] [CrossRef]
- Zhang, R.; Mark, J.E.; Pinhas, A.R. Dehydrocoupling polymerization of bis-silanes and disilanols to poly(silphenylenesiloxane) as catalyzed by rhodium complexes. Macromolecules 2000, 33, 3508–3510. [Google Scholar] [CrossRef]
- Utracki, L.A.; Groeninckx, G. Polymer Blends Handbook. C. Wilkie; Springer: Dordrecht, The Netherlands, 2002; Volume 1, pp. 171–289. ISBN 978-94-007-6065-3. [Google Scholar]
- Houghton, A.Y.; Hurmalainen, J.; Mansikkamäki, A.; Piers, W.E.; Tuononen, H.M. Direct observation of a borane-silane complex involved in frustrated lewis-pair-mediated hydrosilylations. Nat. Chem. 2014, 6, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, R.C.; Grande, J.B.; Brook, M.A.; Adronov, A. Functionalization of single-walled carbon nanotubes via the piers-rubinsztajn reaction. Macromolecules 2014, 47, 6527–6530. [Google Scholar] [CrossRef]
- Dudziec, B.; Rzonsowska, M.; Marciniec, B.; Brząkalski, D.; Woźniak, B. New mono- and diethynylsiloxysilsesquioxanes—Efficient procedures for their synthesis. Dalton Trans. 2014, 43, 13201–13207. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.B.; Urlich, T.; Dickie, T.; Brook, M.A. Silicone dendrons and dendrimers from orthogonal sih coupling reactions. Polym. Chem. 2014, 5, 6728–6739. [Google Scholar] [CrossRef]
- Thompson, D.B.; Brook, M.A. Rapid assembly of complex 3d siloxane architectures. J. Am. Chem. Soc. 2008, 130, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P. Synthesis, characterization and thermal stability of new dumbbell-shaped isobutyl-substituted posss linked by aromatic bridges. J. Therm. Anal. Calorim. 2014, 117, 243–250. [Google Scholar] [CrossRef]
- Gusev, D.; Llamazares, A.; Artus, G.; Heiko Jacobsen, A.; Berke, H. Classical and nonclassical nitrosyl hydride complexes of rhenium in various oxidation states. Organometallics 1999, 18, 75–89. [Google Scholar] [CrossRef]
- Laengert, S.E.; Schneider, A.F.; Chen, Y.; Brook, M.A. Sequential functionalization of a natural crosslinker leads to designer silicone networks. Chem. Asian J. 2017, 12, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztajn, S.; Cella, J.A. A new polycondensation process for the preparation of polysiloxane copolymers. Macromolecules 2005, 38, 1061–1063. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y. Cyclic polysiloxanes with linked rings. Angew. Chem. Int. Ed. 2017, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.K.; Rosenberg, L. Scope and selectivity of b(c6f5)(3)-catalyzed reactions of the disilane (ph2sih)(2). J. Organomet. Chem. 2016, 809, 86–93. [Google Scholar] [CrossRef]
- Casserly, T.B.; Gleason, K.K. Density functional theory calculation of 29si nmr chemical shifts of organosiloxanes. J. Phys. Chem. B 2005, 109, 13605–13610. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yu, J.; Li, Q.; Liu, Y. High molecular weight cyclic polysiloxanes from organocatalytic zwitterionic polymerization of constrained spirocyclosiloxanes. Polym. Chem. 2017, 8, 7301–7306. [Google Scholar] [CrossRef]
- Wang, J.; Du, M.; Xu, C.; Zhu, H.; Fu, Y. Synthesis of transparent densely crosslinked polysiloxane with high refractive index. J. Macromol. Sci. Part B Phys. 2012, 51, 2462–2472. [Google Scholar] [CrossRef]
- Patel, M.; Murphy, J.J.; Skinner, A.R.; Powell, S.J.; Smith, P.F. Volatile evolution from room temperature cured polysiloxane rubber induced by irradiation with he2+ ions. Polym. Test. 2003, 22, 923–928. [Google Scholar] [CrossRef]
- Chien, A.; Maxwell, R.; Chambers, D.; Balazs, B.; Lemay, J. Characterization of radiation-induced aging in silica-reinforced polysiloxane composites. Radiat. Phys. Chem. 2000, 59, 493–500. [Google Scholar] [CrossRef]







| No. | Temperature (°C) | Catalyst Concentration (mmol·L−1) | Reactant Concentration (wt %) | Yield (%) | Mw (KDa) | Mw/Mn |
|---|---|---|---|---|---|---|
| B01 | 25 | 0.8 | 35 | 71.42 | 26.13 | 3.57 |
| B02 | 25 | 0.4 | 35 | 79.00 | 11.64 | 2.11 |
| B03 | 25 | 0.2 | 35 | 79.48 | 7.39 | 1.68 |
| B04 | 25 | 0.1 | 35 | 86.48 | 6.10 | 1.52 |
| B05 | 0 | 0.8 | 35 | 72.32 | 32.17 | 3.83 |
| B06 | 10 | 0.8 | 35 | 78.30 | 65.09 | 7.08 |
| B07 | 40 | 0.8 | 35 | 76.74 | 20.63 | 3.17 |
| B08 | 60 | 0.8 | 35 | 73.57 | 14.38 | 2.50 |
| B09 | 25 | 0.1 | 65 | gel | / | / |
| B10 | 25 | 0.1 | 50 | gel | / | / |
| B11 | 25 | 0.1 | 20 | 80.60 | 8.78 | 2.01 |
| D01 | 25 | 0.8 | 35 | 60.60 | 40.52 | 4.76 |
| D02 | 25 | 0.4 | 35 | 62.74 | 9.10 | 3.73 |
| D03 | 25 | 0.2 | 35 | 74.24 | 7.25 | 3.88 |
| D04 | 25 | 0.1 | 35 | 78.68 | 4.77 | 3.85 |
| D05 | 0 | 0.8 | 35 | 72.19 | 48.03 | 4.50 |
| D06 | 10 | 0.8 | 35 | 63.29 | 75.60 | 5.37 |
| D07 | 40 | 0.8 | 35 | 73.47 | 34.36 | 3.28 |
| D08 | 60 | 0.8 | 35 | 73.57 | 22.52 | 4.72 |
| D09 | 25 | 0.1 | 65 | gel | / | / |
| D10 | 25 | 0.1 | 50 | gel | / | / |
| D11 | 25 | 0.1 | 20 | 83.82 | 5.77 | 1.53 |
| Species | Bond Length (Å) | Atomic Charge | Species | Bond Length (Å) | Atomic Charge |
|---|---|---|---|---|---|
| DPDMS | RO(24)-C(26) = 1.4249 | qSi(1) = 0.7899 | DPDES | RO(24)-C(29) = 1.4306 | qSi(1) = 1.1188 |
| RO(25)-C(30) = 1.4249 | qO(24) = −0.4336 | RO(25)-C(26) = 1.4307 | qO(24) = −0.3791 | ||
| RO(24)-Si(1) = 1.666 | qO(25) = −0.4335 | RO(24)-Si(1) = 1.6628 | qO(25) = −0.3789 | ||
| RO(25)-Si(1) = 1.666 | qC(26) = 0.2844 | RO(25)-Si(1) = 1.6628 | qC(26) = 0.2034 | ||
| qC(30) = 0.2844 | qC(29) = 0.2035 | ||||
| miniHCS /B(C6F5)3 | RH(37)-Si(36) = 1.4474 | qB = 0.2745 | miniHCS | RH(3)-Si(2) = 1.4523 | qSi(2) = 0.7965 |
| RH(38)-Si(36) = 1.4546 | qSi(36) = 0.5359 | RH(4)-Si(2) = 1.453 | qSi(5) = 0.7940 | ||
| RH(40)-Si(39) = 1.4476 | qSi(39) = 0.5743 | RH(6)-Si(5) = 1.4531 | qH(3) = −0.1519 | ||
| RH(41)-Si(39) = 1.4492 | qH(37) = −0.1314 | RH(7)-Si(5) = 1.4527 | qH(4) = −0.1499 | ||
| qH(38) = −0.1502 | qH(6) = −0.1447 | ||||
| qH(40) = −0.1153 | qH(7) = −0.1507 | ||||
| qH(41) = −0.1149 | B(C6F5)3 | qB = 0.802 |
| No. | Refractive Index | T5% (°C) | T10% (°C) | RW (%) |
|---|---|---|---|---|
| HCS | 1.4123 | 141.5 | 229.3 | 14.6 |
| B01 | 1.5190 | 309.8 | 374.5 | 72.4 |
| B02 | 1.5189 | 302 | 362.2 | 72.32 |
| B03 | 1.5186 | 290.3 | 342.7 | 65.43 |
| B04 | 1.5078 | 270.8 | 332.7 | 68.74 |
| B05 | 1.5191 | 324 | 380.8 | 75.60 |
| B06 | 1.5190 | 316.8 | 368 | 65.54 |
| B07 | 1.5163 | 263 | 335.8 | 70.38 |
| B08 | 1.5151 | 233.7 | 306.5 | 66.66 |
| B09 | — | — | — | — |
| B10 | — | — | — | — |
| B11 | 1.5056 | 265 | 369.3 | 71.47 |
| D01 | 1.5173 | 284.7 | 351.8 | 68.94 |
| D02 | 1.5160 | 265.5 | 319.7 | 64.66 |
| D03 | 1.5142 | 260.7 | 339.3 | 72.51 |
| D04 | 1.5119 | 235.2 | 309.5 | 68.99 |
| D05 | 1.5162 | 280.5 | 371.7 | 75.92 |
| D06 | 1.5168 | 273.2 | 356.2 | 72.81 |
| D07 | 1.5159 | 250.2 | 327 | 73.92 |
| D08 | 1.5151 | 217.5 | 272.8 | 59.96 |
| D09 | — | — | — | — |
| D10 | — | — | — | — |
| D11 | 1.5159 | 203.7 | 259 | 58.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, M.; Chen, X.; Wu, S.; Ge, J.; Zhou, X.; Yin, G. Fabrication of Reactive Poly(Phenyl-Substituted Siloxanes/Silsesquioxanes) with Si‒H and Alkoxy Functional Groups via the Piers–Rubinsztajn Reaction. Polymers 2018, 10, 1006. https://doi.org/10.3390/polym10091006
Yi M, Chen X, Wu S, Ge J, Zhou X, Yin G. Fabrication of Reactive Poly(Phenyl-Substituted Siloxanes/Silsesquioxanes) with Si‒H and Alkoxy Functional Groups via the Piers–Rubinsztajn Reaction. Polymers. 2018; 10(9):1006. https://doi.org/10.3390/polym10091006
Chicago/Turabian StyleYi, Minghao, Xunjun Chen, Shufang Wu, Jianfang Ge, Xinhua Zhou, and Guoqiang Yin. 2018. "Fabrication of Reactive Poly(Phenyl-Substituted Siloxanes/Silsesquioxanes) with Si‒H and Alkoxy Functional Groups via the Piers–Rubinsztajn Reaction" Polymers 10, no. 9: 1006. https://doi.org/10.3390/polym10091006
APA StyleYi, M., Chen, X., Wu, S., Ge, J., Zhou, X., & Yin, G. (2018). Fabrication of Reactive Poly(Phenyl-Substituted Siloxanes/Silsesquioxanes) with Si‒H and Alkoxy Functional Groups via the Piers–Rubinsztajn Reaction. Polymers, 10(9), 1006. https://doi.org/10.3390/polym10091006