Photoinitiator Free Resins Composed of Plant-Derived Monomers for the Optical µ-3D Printing of Thermosets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Real-Time Photorheometry
2.3. Preparation of Cross-Linked Polymers
2.4. Chemical Structure Analysis
2.5. Soxhlet Extraction
2.6. Differential Scanning Calorimetry
2.7. Thermogravimetric Analysis
2.8. Mechanical Testing
2.9. Direct Laser Writing 3D Lithography
3. Results
3.1. Kinetics of Photocross-Linking
3.2. Characterization of Photocross-Linked Polymer Structure
3.3. Thermal Properties
3.4. Compressive Modulus
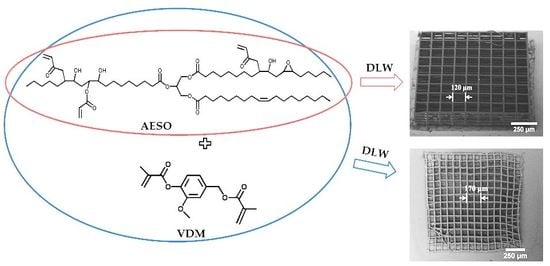
3.5. Characterization of DLW 3D Lithography Produced Structures
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.; Giesel, F.L. 3D Printing Based on Imaging Data: Review of Medical Applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.N.; Fisher, J.P.; Dean, D.; Rimnac, C.; Mikos, A.G. Use of Stereolithography to Manufacture Critical-sized 3D Biodegradable Scaffolds for Bone Ingrowth. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2003, 64, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Corbel, S.; Dufaud, O.; Roques-Carmes, T. Materials for stereolithography. In Stereolithography; Springer: Boston, MA, USA, 2011; pp. 141–159. [Google Scholar]
- Skliutas, E.; Kasetaite, S.; Jonušauskas, L.; Ostrauskaite, J.; Malinauskas, M. Photosensitive Naturally Derived Resins toward Optical 3-D Printing. Opt. Eng. 2018, 57, 041412. [Google Scholar] [CrossRef]
- Ligon-Auer, S.C.; Schwentenwein, M.; Gorsche, C.; Stampfl, J.; Liska, R. Toughening of Photo-Curable Polymer Networks: A Review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased Plastics and Bionanocomposites: Current Status and Future Opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Güner, F.S.; Yağcı, Y.; Erciyes, A.T. Polymers from Triglyceride Oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galia, M.; Cádiz, V. Plant Oils as Platform Chemicals for Polyurethane Synthesis: Current State-of-the-Art. Biomacromolecules 2010, 11, 2825–2835. [Google Scholar] [CrossRef]
- Pelletier, H.; Gandini, A. Preparation of Acrylated and Urethanated Triacylglycerols. Eur. J. Lipid Sci. Technol. 2006, 108, 411–420. [Google Scholar] [CrossRef]
- Pelletier, H.; Belgacem, N.; Gandini, A. Acrylated Vegetable Oils as Photocrosslinkable Materials. J. Appl. Polym. Sci. 2006, 99, 3218–3221. [Google Scholar] [CrossRef]
- Wool, R.; Sun, X.S. Bio-Based Polymers and Composites; Elsevier: Burlington, MA, USA, 2011. [Google Scholar]
- Ivanov, D.S.; Lević, J.D.; Sredanović, S.A. Fatty Acid Composition of various Soybean Products. Food Feed Res. 2010, 37, 65–70. [Google Scholar]
- Haun, W.; Coffman, A.; Clasen, B.M.; Demorest, Z.L.; Lowy, A.; Ray, E.; Retterath, A.; Stoddard, T.; Juillerat, A.; Cedrone, F. Improved Soybean Oil Quality by Targeted Mutagenesis of the Fatty Acid Desaturase 2 Gene Family. Plant Biotechnol. J. 2014, 12, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B. Thiol–ene “click” Reactions and Recent Applications in Polymer and Materials Synthesis: A First Update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Huang, X.; Liu, H.; Shang, S.; Rao, X.; Song, J. Preparation and Characterization of Polymeric Surfactants Based on Epoxidized Soybean Oil Grafted Hydroxyethyl Cellulose. J. Agric. Food Chem. 2015, 63, 9062–9068. [Google Scholar] [CrossRef]
- Liu, Z.; Doll, K.M.; Holser, R.A. Boron Trifluoride Catalyzed Ring-Opening Polymerization of Epoxidized Soybean Oil in Liquid Carbon Dioxide. Green Chem. 2009, 11, 1774–1780. [Google Scholar] [CrossRef]
- Fu, L.; Yang, L.; Dai, C.; Zhao, C.; Ma, L. Thermal and Mechanical Properties of Acrylated Expoxidized-soybean Oil-based Thermosets. J. Appl. Polym. Sci. 2010, 117, 2220–2225. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Meier, M.A. Plant Oils: The Perfect Renewable Resource for Polymer Science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Sacristán, M.; Ronda, J.C.; Galià, M.; Cádiz, V. Synthesis and Properties of Boron-Containing Soybean Oil Based Thermosetting Copolymers. Polymer 2010, 51, 6099–6106. [Google Scholar] [CrossRef]
- Del Rio, E.; Lligadas, G.; Ronda, J.; Galia, M.; Cadiz, V. Biobased Polyurethanes from Polyether Polyols obtained by Ionic-coordinative Polymerization of Epoxidized Methyl Oleate. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5009–5017. [Google Scholar] [CrossRef]
- Del Rio, E.; Galia, M.; Cadiz, V.; Lligadas, G.; Ronda, J. Polymerization of Epoxidized Vegetable Oil Derivatives: Ionic-coordinative Polymerization of Methylepoxyoleate. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4995–5008. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, M.; Huang, X.; Zhang, H.; Shang, S.; Song, J. Synthesis and Performance of a Thermosetting Resin: Acrylated Epoxidized Soybean Oil Curing with a Rosin-based Acrylamide. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Demengeot, E.; Baliutaviciene, I.; Ostrauskaite, J.; Augulis, L.; Grazuleviciene, V.; Rageliene, L.; Grazulevicius, J.V. Crosslinking of Epoxidized Natural Oils with Diepoxy Reactive Diluents. J. Appl. Polym. Sci. 2010, 115, 2028–2038. [Google Scholar] [CrossRef]
- Yadav, S.K.; Schmalbach, K.M.; Kinaci, E.; Stanzione, J.F.; Palmese, G.R. Recent Advances in Plant-Based Vinyl Ester Resins and Reactive Diluents. Eur. Polym. J. 2018, 98, 199–215. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, H.R.; Kim, B.S. Soybean Oil-Based Photo-Crosslinked Polymer Networks. J. Polym. Environ. 2010, 18, 291–297. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.F.; Fernando, S.; Jagodzinski, K. Soy-Based, High Biorenewable Content UV Curable Coatings. Prog. Org. Coat. 2011, 71, 98–109. [Google Scholar] [CrossRef]
- Wu, J.F.; Fernando, S.; Jagodzinski, K.; Weerasinghe, D.; Chen, Z. Effect of Hyperbranched Acrylates on UV-curable Soy-based Biorenewable Coatings. Polym. Int. 2011, 60, 571–577. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Xia, J.; Song, J.; Huang, K.; Li, M. Renewable Myrcene-Based UV-Curable Monomer and Its Copolymers with Acrylated Epoxidized Soybean Oil: Design, Preparation, and Characterization. BioResources 2015, 10, 2130–2142. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Patani, R.; Wu, J.F.; Fernando, S.; Jogodzinski, K.; Webster, D.C. Soy-Based UV-Curable Thiol–ene Coatings. J. Coat. Technol. Res. 2010, 7, 603–613. [Google Scholar] [CrossRef]
- Kašėtaitė, S.; De la Flor, S.; Serra, A.; Ostrauskaitė, J. Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers. Polymers 2018, 10, 439. [Google Scholar] [CrossRef]
- Can, E.; Wool, R.; Küsefoğlu, S. Soybean-and Castor-oil-based Thermosetting Polymers: Mechanical Properties. J. Appl. Polym. Sci. 2006, 102, 1497–1504. [Google Scholar] [CrossRef]
- Lu, J.; Khot, S.; Wool, R.P. New Sheet Molding Compound Resins from Soybean Oil. I. Synthesis and Characterization. Polymer 2005, 46, 71–80. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Corn Oil-based Composites Reinforced with Continuous Glass Fibers: Fabrication and Properties. J. Appl. Polym. Sci. 2006, 102, 3345–3353. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Novel Biobased Nanocomposites from Soybean Oil and Functionalized Organoclay. Biomacromolecules 2006, 7, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, D.D.; Larock, R.C. Novel Rubbers from Cationic Copolymerization of Soybean Oils and Dicyclopentadiene. 1. Synthesis and Characterization. Biomacromolecules 2006, 7, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.; Scala, J.J.L.; Wool, R. Fatty Acid-based Comonomers as Styrene Replacements in Soybean and Castor Oil-based Thermosetting Polymers. J. Appl. Polym. Sci. 2011, 119, 1000–1010. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Divinylbenzene-HP (Cas no. 1321-74-0) in F344/N Rats and B6C3F1 Mice (Inhalation Studies). Natl. Toxicol. Program. Tech. Rep. Ser. 2006, 534, 1–290. [Google Scholar]
- Roe, F.J. Styrene: Toxicity Studies—What do they show? Crit. Rev. Toxicol. 1994, 24, s117–s125. [Google Scholar] [CrossRef]
- Kinkead, E.; Pozzani, U.; Geary, D.; Carpenter, C. The Mammalian Toxicity of Dicyclopentadiene. Toxicol. Appl. Pharmacol. 1971, 20, 552–561. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and its use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Renewable Polymers Prepared from Vanillin and Its Derivatives. Macromol. Chem. Phys. 2015, 216, 1816–1822. [Google Scholar] [CrossRef]
- Zhang, Y.; Thakur, V.K.; Li, Y.; Garrison, T.F.; Gao, Z.; Gu, J.; Kessler, M.R. Soybean-Oil-Based Thermosetting Resins with Methacrylated Vanillyl Alcohol as Bio-Based, Low-Viscosity Comonomer. Macromol. Mater. Eng. 2018, 303, 1700278. [Google Scholar] [CrossRef]
- Schmidt, L.E.; Leterrier, Y.; Vesin, J.; Wilhelm, M.; Månson, J.E. Photorheology of Fast UV-Curing Multifunctional Acrylates. Macromol. Mater. Eng. 2005, 290, 1115–1124. [Google Scholar] [CrossRef]
- Schmidt, L.E.; Schmäh, D.; Leterrier, Y.; Månson, J.E. Time-Intensity Transformation and Internal Stress in UV-Curable Hyperbranched Acrylates. Rheol. Acta 2007, 46, 693–701. [Google Scholar] [CrossRef]
- Rekštytė, S.; Jonavičius, T.; Malinauskas, M. Direct Laser Writing of Microstructures on Optically Opaque and Reflective Surfaces. Opt. Lasers Eng. 2014, 53, 90–97. [Google Scholar] [CrossRef]
- Kasetaite, S.; Ostrauskaite, J.; Grazuleviciene, V.; Bridziuviene, D.; Budreckiene, R.; Rainosalo, E. Biodegradable Photocross-Linked Polymers of Glycerol Diglycidyl Ether and Structurally Different Alcohols. React. Funct. Polym. 2018, 122, 42–50. [Google Scholar] [CrossRef]
- Borges, A.; Bourban, P.; Pioletti, D.; Månson, J. Curing Kinetics and Mechanical Properties of a Composite Hydrogel for the Replacement of the Nucleus Pulposus. Compos. Sci. Technol. 2010, 70, 1847–1853. [Google Scholar] [CrossRef]
- Sarmento, V.; Frigerio, M.; Dahmouche, K.; Pulcinelli, S.H.; Santilli, C.V. Evolution of Rheological Properties and Local Structure during Gelation of Siloxane-Polymethylmethacrylate Hybrid Materials. J. Sol. Gel. Sci. Technol. 2006, 37, 179–184. [Google Scholar] [CrossRef]
- Anseth, K.S.; Wang, C.M.; Bowman, C.N. Reaction Behaviour and Kinetic Constants for Photopolymerizations of Multi (Meth) Acrylate Monomers. Polymer 1994, 35, 3243–3250. [Google Scholar] [CrossRef]
- Kreutzer, J.; Qin, X.; Gorsche, C.; Peterlik, H.; Liska, R.; Schubert, U. Variation of the Crosslinking Density in Cluster-Reinforced Polymers. Mater. Today Commun. 2015, 5, 10–17. [Google Scholar] [CrossRef]
- Beyler, C.L.; Hirschler, M.M. Thermal Decomposition of Polymers. SFPE Handb. Fire Prot. Eng. 2002, 2, 111–131. [Google Scholar]
- Kasetaite, S.; Ostrauskaite, J.; Grazuleviciene, V.; Svediene, J.; Bridziuviene, D. Camelina Oil-and Linseed Oil-based Polymers with Bisphosphonate Crosslinks. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Nagai, A.; Kamei, Y.; Wang, X.; Omura, M.; Sudo, A.; Nishida, H.; Kawamoto, E.; Endo, T. Synthesis and Crosslinking Behavior of a Novel Linear Polymer Bearing 1, 2, 3-triazol and Benzoxazine Groups in the Main Chain by a Step-growth Click-coupling Reaction. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2316–2325. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Bičkauskaitė, G.; Gadonas, R.; Juodkazis, S. Mechanisms of Three-Dimensional Structuring of Photo-Polymers by Tightly Focussed Femtosecond Laser Pulses. Opt. Express 2010, 18, 10209–10221. [Google Scholar] [CrossRef] [PubMed]
- Parkatzidis, K.; Kabouraki, E.; Selimis, A.; Kaliva, M.; Ranella, A.; Farsari, M.; Vamvakaki, M. Initiator-Free, Multiphoton Polymerization of Gelatin Methacrylamide. Macromol. Mater. Eng. 2018, 303, 1800458. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast Laser Processing of Materials: From Science to Industry. Light Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef] [PubMed]
- Jonušauskas, L.; Gailevičius, D.; Rekštytė, S.; Baldacchini, T.; Juodkazis, S.; Malinauskas, M. Mesoscale Laser 3D Printing. Preprints 2018, 2018100384. [Google Scholar] [CrossRef]
- Jonušauskas, L.; Gailevičius, D.; Mikoliūnaitė, L.; Sakalauskas, D.; Šakirzanovas, S.; Juodkazis, S.; Malinauskas, M. Optically Clear and Resilient Free-Form µ-Optics 3D-Printed via Ultrafast Laser Lithography. Materials 2017, 10, 12. [Google Scholar] [CrossRef] [PubMed]








| Resin | Ratio of Functional Groups 1 | Ratio of Monomers, mol | Amount of Monomers, g |
|---|---|---|---|
| AESO | - | 1 | 1.5 |
| AESO/VDM1 | 1.5:1 | 1:1 | 1.5/0.375 |
| AESO/VDM2 | 3:1 | 1:0.5 | 1.5/0.187 |
| AESO/VDM3 | 6:1 | 1:0.25 | 1.5/0.094 |
| AESO/VDA1 | 1.5:1 | 1:1 | 1.5/0.339 |
| AESO/VDA2 | 3:1 | 1:0.5 | 1.5/0.170 |
| AESO/VDA3 | 6:1 | 1:0.25 | 1.5/0.085 |
| Resin | G’ 1, Pa | G” 1, Pa | η* 1, mPa·s | tgel, s 2 |
|---|---|---|---|---|
| AESO | 4.76 × 106 | 1.47 × 106 | 7.93 × 104 | 49 |
| AESO/VDM1 | 2.65 × 106 | 1.01 × 106 | 4.51 × 104 | 98 |
| AESO/VDM2 | 2.70 × 106 | 9.31 × 105 | 4.55 × 104 | 91 |
| AESO/VDM3 | 3.51 × 106 | 1.18 × 106 | 5.89 × 104 | 66 |
| AESO/VDA1 | 5.89 × 105 | 2.27 × 105 | 1.01 × 104 | 782 |
| AESO/VDA2 | 1.11 × 106 | 3.74 × 105 | 1.87 × 104 | 531 |
| AESO/VDA3 | 3.56 × 106 | 1.17 × 106 | 5.96 × 104 | 290 |
| Polymer | Yield of Insoluble Fraction 1 (%) | Tg 2 (°C) | Tdec-10% 3 (°C) | Ec 4 (Pa) |
|---|---|---|---|---|
| pAESO | 88 | −4.5 | 356 | 0.62 ± 0.1 |
| pAESO/VDM1 | 31 | −2.6 | 295 | 0.19 ± 0.03 |
| pAESO/VDM2 | 48 | −2.6 | 318 | 0.46 ± 0.13 |
| pAESO/VDM3 | 63 | −1.6 | 331 | 0.66 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedevaite, M.; Ostrauskaite, J.; Skliutas, E.; Malinauskas, M. Photoinitiator Free Resins Composed of Plant-Derived Monomers for the Optical µ-3D Printing of Thermosets. Polymers 2019, 11, 116. https://doi.org/10.3390/polym11010116
Lebedevaite M, Ostrauskaite J, Skliutas E, Malinauskas M. Photoinitiator Free Resins Composed of Plant-Derived Monomers for the Optical µ-3D Printing of Thermosets. Polymers. 2019; 11(1):116. https://doi.org/10.3390/polym11010116
Chicago/Turabian StyleLebedevaite, Migle, Jolita Ostrauskaite, Edvinas Skliutas, and Mangirdas Malinauskas. 2019. "Photoinitiator Free Resins Composed of Plant-Derived Monomers for the Optical µ-3D Printing of Thermosets" Polymers 11, no. 1: 116. https://doi.org/10.3390/polym11010116
APA StyleLebedevaite, M., Ostrauskaite, J., Skliutas, E., & Malinauskas, M. (2019). Photoinitiator Free Resins Composed of Plant-Derived Monomers for the Optical µ-3D Printing of Thermosets. Polymers, 11(1), 116. https://doi.org/10.3390/polym11010116