The Investigation of the Seebeck Effect of the Poly(3,4-Ethylenedioxythiophene)-Tosylate with the Various Concentrations of an Oxidant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PEDOT-Tos
2.2. Characterizations
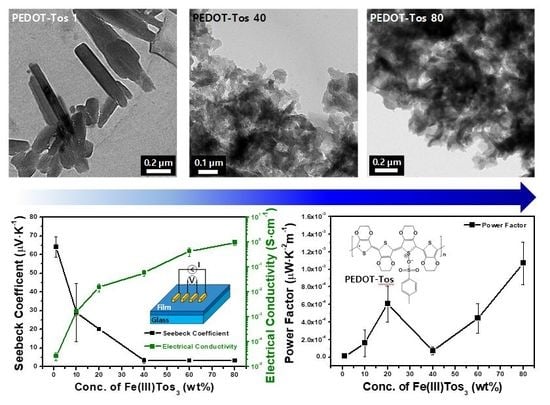
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, B.; Xu, J.-H.; Uher, C.; Morelli, D.T.; Meisner, G.P.; Fleurial, J.-P.; Caillat, T.; Borshchevsky, A. Low-temperature transport properties of the filled skutterudites CeFe4−xCoxSb12s. Phys. Rev. B 1997, 55, 1476. [Google Scholar] [CrossRef]
- Uher, C.; Yang, J.; Hu, S.; Morelli, D.T.; Meisner, G.P. High temperature thermoelectric properties of MNiSn (M=Zr, Hf). Phys. Rev. B 1999, 59, 8615. [Google Scholar] [CrossRef]
- Nolas, G.S.; Cohn, J.L.; Slack, G.A.; Schujman, S.B. Semiconducting Ge clathrates: Promising candidates for thermoelectric applications. Appl. Phys. Lett. 1998, 73, 178. [Google Scholar] [CrossRef]
- Littleton, R.T.; Tritt, T.M.; Feger, C.R.; Kolis, J.; Wilson, M.L.; Marone, M.; Payne, J.; Verebeli, D.; Levy, F. Effect of Ti substitution on the thermoelectric properties of the pentatelluride materials M1-xTixTe5 (M=Hf, Zr). Appl. Phys. Lett. 1998, 72, 2056. [Google Scholar] [CrossRef]
- Nolas, G.S.; Morelli, D.T.; Tritt, T.M. SKUTTERUDITES: A Phonon-Glass-Electron Crystal Approach to Advanced Thermoelectric Energy Conversion Applications. Annu. Rev. Mater. Sci. 1999, 29, 89. [Google Scholar] [CrossRef]
- Zebarjadi, M.; Esfarjani, K.; Dresselhaus, M.S.; Ren, Z.F.; Chen, G. Perspectives on thermoelectrics: From fundamentals to device applications. Energy Environ. Sci. 2012, 5, 5147. [Google Scholar] [CrossRef]
- Dubey, N.; Leclerc, M. Conducting polymers: Efficient thermoelectric materials. J. Polym. Sci. B Polym. Phys. 2011, 49, 467. [Google Scholar] [CrossRef]
- Huewe, F.; Steeger, A.; Kostova, K.; Burroughs, L.; Bauer, I.; Strohriegl, P.; Dimitrov, V.; Woodward, S.; Pflaum, J. Low-cost and sustainable organic thermoelectrics based on low-dimensional molecular metals. Adv. Mater. 2017, 29, 1605682. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Huang, D.; Meng, Q.; Di, C.A.; Zhu, D. Correlation between Seebeck coefficient and transport energy level in poly(3-hexylthiophene). Organ. Electron. 2018, 56, 125. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.M.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.; Paul, B.; Eklund, P. Flexible thermoelectric materials and devices. Appl. Mater. Today 2018, 12, 366. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Recent Progress on PEDOT-based thermoelectric materials. Materials 2015, 8, 732. [Google Scholar] [CrossRef] [PubMed]
- Bahk, J.-H.; Fang, H.; Yazawa, K.; Shakouri, A. Flexible thermoelectric materials and device optimization for wearable energy harvesting. J. Mater. Chem. C 2015, 3, 10362. [Google Scholar] [CrossRef]
- Du, Y.; Shen, S.Z.; Cai, K.; Casey, P.S. Research progress on polymer-inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 2012, 37, 820. [Google Scholar] [CrossRef]
- Sun, J.; Yeh, M.-L.; Jung, B.J.; Zhang, B.; Feser, J.; Majumdar, A.; Katz, H.E. Simultaneous increase in seebeck coefficient and conductivity in a doped poly(alkylthiophene) blend with defined density of states. Macromolecules 2010, 43, 2897. [Google Scholar] [CrossRef]
- Kirchmeyer, S.; Reuter, K. Scientific importance, properties and growing applications of poly(3,4-ethylenedioxythiophene). J. Mater. Chem. 2005, 15, 2077. [Google Scholar] [CrossRef]
- Louwet, F.; Groenendaal, L.; Dhaen, J.; Manca, J.; Luppen, J.V.; Verdonck, E.; Leenders, L. PEDOT/PSS: Synthesis, characterization, properties and applications. Synth. Met. 2003, 135–136, 115. [Google Scholar] [CrossRef]
- Chang, K.-C.; Jeng, M.-S.; Yang, C.-C.; Chou, Y.-W.; Wu, S.-K.; Thomas, M.A.; Peng, Y.-C. The Thermoelectric Performance of Poly(3,4-ethylenedi oxythiophene)/Poly(4-styrenesulfonate) Thin Films. J. Electron. Mater. 2009, 38, 1182. [Google Scholar] [CrossRef]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, F.; Huang, M.; Yue, R.; Lu, B.; Xu, J.; Liu, G. Thermoelectric performance of poly(3,4-Ethylenedioxy-thiophene)/poly(Styrenesulfonate) pellets and films. J. Electron. Mater. 2011, 40, 648. [Google Scholar] [CrossRef]
- See, K.C.; Feser, J.P.; Chen, C.E.; Majumder, A.; Urban, J.J.; Segalman, R.A. Water-processable polymer-nanocrystal hybrids for thermoelectrics. Nano Lett. 2010, 10, 4664. [Google Scholar] [CrossRef] [PubMed]
- Levermore, P.; Chen, L.; Wang, X.; Das, R.; Bradley, D. Fabrication of highly conductive poly(3,4-ethylenedioxythiophene) films by vapor phase polymerization and their application in efficient organic light-emitting diodes. Adv. Mater. 2007, 19, 2379. [Google Scholar] [CrossRef]
- Ha, Y.-H.; Nikolov, N.; Pollack, S.K.; Mastrangelo, J.; Martin, B.D.; Shashidhar, R. Towards a transparent, highly conductive poly(3,4-ethylenedioxythiophene). Adv. Funct. Mater. 2004, 14, 615. [Google Scholar] [CrossRef]
- Fewings, K.; Junk, P.; Georganopoulou, D.; Prince, P.; Steed, J. Supramolecular interactions in metal tosylate complexes. Polyhedron 2001, 20, 643. [Google Scholar] [CrossRef]
- Chen, J.; Winther-Jensen, B.; Pornputtkul, Y.; West, K.; Kane-Maquarie, L.; Wallace, G.G. Synthesis of chiral polyaniline films via chemical vapor phase polymerization. Electrochem. Solid-State Lett. 2006, 9, C9. [Google Scholar] [CrossRef]
- Shi, W.; Yao, Q.; Qu, S.; Chen, H.; Zhang, T.; Chen, L. Micron-thick highly conductive PEDOT films synthesized via self-inhibited polymerization: Roles of anions. NPG Asia Mater. 2017, 9, e405. [Google Scholar] [CrossRef]
- Schumacher, B.; Bach, H.-G.; Spitzer, P.; Obrzut, J. “Electrical Properties” in Springer Handbook of Materials Measurement Methods; Czichos, H., Saito, T., Smith, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 431. [Google Scholar]
- Lim, J.A.L.; Park, S.H.; Baek, J.H.; Ko, Y.D.; Lee, H.S.; Cho, K.; Lee, J.Y.; Lee, D.R.; Cho, J.H. Selectively patterned highly conductive poly(3,4-ethylenedioxythiophene)-tosylate electrodes for high performance organic field-effect transistors. Appl. Phys. Lett. 2009, 95, 233509. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.B.; Perepichka, D.F. A new simple synthesis of poly(thiophene-methine)s. Chem. Commun. 2005, 33, 4187. [Google Scholar] [CrossRef]
- Groenedaal, L.B.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reyonds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481. [Google Scholar] [CrossRef]
- Jonas, F.; Schrader, L. Conductive modifications of polymers with polypyrroles and polythiophenes. Synth. Met. 1991, 41–43, 831–836. [Google Scholar] [CrossRef]
- Meng, H.; Perepichka, D.F.; Bendikov, M.; Wudl, F.; Pan, G.Z.; Yu, W.; Dong, W.; Brown, S. Solid-State Synthesis of a Conducting Polythiophene via an Unprecedented Heterocyclic Coupling Reaction. J. Am. Chem. Soc. 2003, 125, 15151. [Google Scholar] [CrossRef] [PubMed]
- Svergun, D. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Cryst. 1992, 25, 495. [Google Scholar] [CrossRef]
- Wu, C.-H.; Dong, T.-M.; Chiu, W.-Y. Characterization and conversion determination of stable PEDOT latex nanoparticles synthesized by emulsion polymerization. Polymer 2011, 52, 1375. [Google Scholar] [CrossRef]
- Fabretto, M.; Zuber, K.; Hall, C.; Murphy, P.; Griesser, H.J. The role of water in the synthesis and performance of vapour phase polymerised PEDOT electrochromic devices. J. Mater. Chem. 2009, 19, 7871. [Google Scholar] [CrossRef]
- Ko, D.-K.; Urban, J.J.; Murray, C.B. Carrier distribution and dynamics of nanocrystal solids doped with artificial atoms. Nano Lett. 2010, 10, 1842. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, H. A general expression for the thermoelectric power. Solid State Commun. 1971, 9, 1813. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Jang, W.; Wang, D.H. The Investigation of the Seebeck Effect of the Poly(3,4-Ethylenedioxythiophene)-Tosylate with the Various Concentrations of an Oxidant. Polymers 2019, 11, 21. https://doi.org/10.3390/polym11010021
Kim J-S, Jang W, Wang DH. The Investigation of the Seebeck Effect of the Poly(3,4-Ethylenedioxythiophene)-Tosylate with the Various Concentrations of an Oxidant. Polymers. 2019; 11(1):21. https://doi.org/10.3390/polym11010021
Chicago/Turabian StyleKim, Joon-Soo, Woongsik Jang, and Dong Hwan Wang. 2019. "The Investigation of the Seebeck Effect of the Poly(3,4-Ethylenedioxythiophene)-Tosylate with the Various Concentrations of an Oxidant" Polymers 11, no. 1: 21. https://doi.org/10.3390/polym11010021
APA StyleKim, J. -S., Jang, W., & Wang, D. H. (2019). The Investigation of the Seebeck Effect of the Poly(3,4-Ethylenedioxythiophene)-Tosylate with the Various Concentrations of an Oxidant. Polymers, 11(1), 21. https://doi.org/10.3390/polym11010021