Flame Inhibition and Charring Effect of Aromatic Polyimide and Aluminum Diethylphosphinate in Polyamide 6
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of API Compound
2.3. Preparation of PA6 Composites
2.4. Characterization
2.4.1. Structural Characterization
2.4.2. Limited Oxygen Index (LOI) Measurement
2.4.3. Vertical Burning Test
2.4.4. Cone Calorimeter Test
2.4.5. Scanning Electron Microscope (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX)
2.4.6. Thermogravimetry—Fourier Transform Infrared Spectroscopy
2.4.7. Tensile and Unnotched Charpy Impact Test
3. Results and Discussion
3.1. Synthesis of API
3.2. Thermal Degradation Behaviors of Flame Retardant PA6 Composites
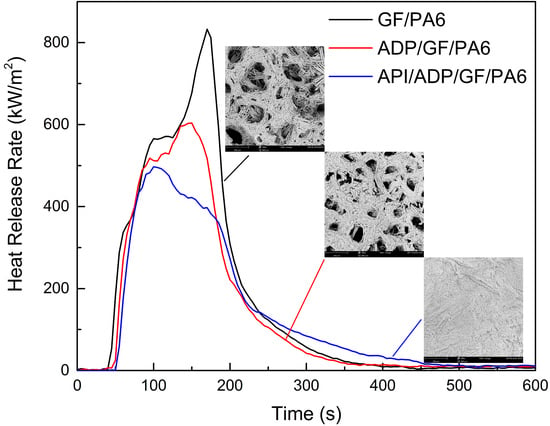
3.3. Flame Retardancy
3.4. Macroscopic and Microscopic Morphologies of Residues
3.5. FTIR Analysis of Residues
3.6. Real-Time TGA-FTIR Analysis on the Evolved Gases from PA6 Composites
3.7. Mechanical Properties of PA6 Composites
4. Conclusions
Supplementary Materials
Author Contributions
Fundings
Conflicts of Interest
References
- Clavería, I.; Elduque, D.; Santolaria, J.; Pina, C.; Javierre, C.; Fernandez, A. The influence of environmental conditions on the dimensional stability of components injected with PA6 and PA66. Polym. Test. 2016, 50, 15–25. [Google Scholar] [CrossRef]
- He, W.T.; Zhu, H.; Zhang, P.C.; Xiang, Y.S.; Zhou, Y.; Qin, S.H.; Yu, J. Pyrolysis and flammability behavior of long (glass fiber)-reinforced polyamide 6 by aluminum alkylphosphinate-based flame retardants in combination with organic montmorillonite. J. Vinyl Addit. Techn. 2018, 24, 27–36. [Google Scholar] [CrossRef]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal decomposition of brominated flame retardants (BFRs): Products and mechanisms. Prog. Energy Combust. Sci. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Altarawneh, M.; Dlugogorski, B.Z. Formation of polybrominated dibenzofurans from polybrominated biphenyls. Chemosphere 2015, 119, 1048–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altarawneh, M.; Dlugogorski, B.Z. Thermal decomposition of 1,2-Bis(2,4,6-tribromophenoxy)ethane (BTBPE), a novel brominated flame retardant. Environ. Sci. Technol. 2014, 48, 14335–14343. [Google Scholar] [CrossRef] [PubMed]
- Casetta, M.; Michaux, G.; Ohl, B.; Duquesne, S.; Bourbigot, S. Key role of magnesium hydroxide surface treatment in the flame retardancy of glass fiber reinforced polyamide 6. Polym. Degrad. Stab. 2018, 148, 95–103. [Google Scholar] [CrossRef]
- Batistella, M.A.; Sonnier, R.; Otazaghine, B.; Petter, C.O.; Lopez-Cuesta, J.M. Interactions between kaolinite and phosphinate-based flame retardant in Polyamide 6. Appl. Clay. Sci. 2018, 157, 248–256. [Google Scholar] [CrossRef]
- Coquelle, M.; Casetta, M.; Sun, J.; Gu, X.; Zhang, S.; Bourbigot, S. Flame Retardancy of PA6 Using a Guanidine Sulfamate/Melamine Polyphosphate Mixture. Polymers 2015, 7, 316–332. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, S.F.; Ling, Z.H.; Zhuang, Y.L.; Chen, Z.Y.; Fan, W.C. Preparation and combustion properties of flame retardant nylon 6/montmorillonite nanocomposite. Macromol. Mater. Eng. 2003, 288, 272–276. [Google Scholar] [CrossRef]
- Butnaru, I.; Fernandez-Ronco, M.P.; Czech-Polak, J.; Heneczkowski, M.; Bruma, M.; Gaan, S. Effect of meltable triazine-DOPO additive on rheological, mechanical, and flammability properties of PA6. Polymers 2015, 7, 1541–1563. [Google Scholar] [CrossRef]
- Isbasar, C.; Hacaloglu, J. Investigation of thermal degradation characteristics of polyamide-6 containing melamine or melamine cyanurate via direct pyrolysis mass spectrometry. J. Anal. Appl. Pyrol. 2012, 98, 221–230. [Google Scholar] [CrossRef]
- Si, G.J.; Li, D.X.; You, Y.L.; Hu, X. Investigation of the influence of red phosphorus, expansible graphite and zinc borate on flame retardancy and wear performance of glass fiber reinforced PA6 composites. Polym. Compos. 2017, 38, 2090–2097. [Google Scholar] [CrossRef]
- He, W.T.; Liao, S.T.; Xiang, Y.S.; Long, L.J.; Qin, S.H.; Yu, J. Structure and properties study of PA6 nanocomposites flame retarded by aluminium salt of diisobutylphosphinic acid and different organic montmorillonites. Polymers 2018, 10, 312. [Google Scholar] [CrossRef]
- Cao, Y.F.; Qian, L.J.; Chen, Y.J.; Wang, Z. Synergistic flame-retardant effect of phosphaphenanthrene derivative and aluminum diethylphosphinate in glass fiber reinforced polyamide 66. J. App. Polym. Sci. 2017, 134, 45126. [Google Scholar] [CrossRef]
- Sehic, A.; Tomsic, B.; Jerman, I.; Vasiljevic, J.; Medved, J.; Simoncic, B. Synergistic inhibitory action of P- and Si-containing precursors in sol-gel coatings on the thermal degradation of polyamide 6. Polym. Degrad. Stab. 2016, 128, 245–252. [Google Scholar] [CrossRef]
- Sahyoun, J.; Bounor-Legare, V.; Ferry, L.; Sonnier, R.; Da Cruz-Boisson, F.; Melis, F.; Bonhomme, A.; Cassagnau, P. Synthesis of a new organophosphorous alkoxysilane precursor and its effect on the thermal and fire behavior of a PA66/PA6 copolymer. Eur. Polym. J. 2015, 66, 352–366. [Google Scholar] [CrossRef]
- Xie, M.C.; Zhang, S.M.; Ding, Y.F.; Wang, F.; Liu, P.; Tang, H.Y.; Wang, Y.T.; Yang, M.S. Synthesis of a heat-resistant DOPO derivative and its application as flame-retardant in engineering plastics. J. App. Polym. Sci. 2017, 134, 44892. [Google Scholar] [CrossRef]
- Li, M.L.; Zhong, Y.H.; Wang, Z.; Fischer, A.; Ranft, F.; Drummer, D.; Wu, W. Flame retarding mechanism of Polyamide 6 with phosphorus-nitrogen flame retardant and DOPO derivatives. J. App. Polym. Sci. 2016, 133, 42932. [Google Scholar] [CrossRef]
- Sun, J.; Gu, X.Y.; Zhang, S.; Coquelle, M.; Bourbigot, S.; Duquesne, S.; Casetta, M. Improving the flame retardancy of polyamide 6 by incorporating hexachlorocyclotriphosphazene modified MWNT. Polym. Adv. Technol. 2014, 25, 1099–1107. [Google Scholar] [CrossRef]
- Guo, Z.B.; Wang, C.L.; Li, J.; Yao, Q. Micro-intumescent flame retardant polyamide 6 based on cyclic phosphate grafting phenol formaldehyde. Polym. Adv. Technol. 2016, 27, 955–963. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Jian, R.K.; Wang, Y.Z. Synergistic effect between aluminum hypophosphite and alkyl-substituted phosphinate in flame-retarded polyamide 6. Ind. Eng. Chem. Res. 2013, 52, 17162–17170. [Google Scholar] [CrossRef]
- He, Q.X.; Tang, L.; Fu, T.; Shi, Y.Q.; Wang, X.L.; Wang, Y.Z. Novel phosphorus-containing halogen-free ionic liquids: Effect of sulfonate anion size on physical properties, biocompatibility, and flame retardancy. RSC Adv. 2016, 6, 52485–52494. [Google Scholar] [CrossRef]
- Kundu, C.K.; Wang, X.; Hou, Y.B.; Hu, Y. Construction of flame retardant coating on polyamide 6.6 via UV grafting of phosphorylated chitosan and sol-gel process of organo-silane. Carbohyd. Polym. 2017, 181, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Majka, T.M.; Cokot, M.; Pielichowski, K. Studies on the thermal properties and flammability of polyamide 6 nanocomposites surface-modified via layer-by-layer deposition of chitosan and montmorillonite. J. Therm. Anal. Calorim. 2018, 131, 405–416. [Google Scholar] [CrossRef]
- Shabanian, M.; Kang, N.J.; Wang, D.Y.; Wagenknecht, U.; Heinrich, G. Synthesis, characterization and properties of novel aliphatic-aromatic polyamide/functional carbon nanotube nanocomposites via in situ polymerization. RSC Adv. 2013, 43, 20738–20745. [Google Scholar] [CrossRef]
- Ge, H.; Wang, W.; Pan, Y.; Yu, X.J.; Hu, W.Z.; Hu, Y. An inherently flame-retardant polyamide containing a phosphorus pendent group prepared by interfacial polymerization. RSC Adv. 2016, 6, 81802–81808. [Google Scholar] [CrossRef]
- Kaynak, C.; Polat, O. Influences of nanoclays on the flame retardancy of fiber-filled and unfilled polyamide-6 with and without aluminum diethylphosphinate. J. Fire Sci. 2015, 33, 87–112. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Sun, N. The effect of morphology on the flame-retardant behaviors of melamine cyanurate in PA6 composites. J. App. Polym. Sci. 2014, 131, 40558. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine cyanurate in glass-fibre-Reinforced Poly(1,4-butylene terephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Ma, K.; Li, B.; Xu, M.J. Simultaneously improving the flame retardancy and mechanical properties for polyamide 6/aluminum diethylphosphinate composites by incorporating of 1,3,5-triglycidyl isocyanurate. Polym. Adv. Technol. 2018, 29, 1068–1077. [Google Scholar] [CrossRef]
- Qiu, Y.; Wachtendorf, V.; Klack, P.; Qian, L.J.; Liu, Z.; Schartel, B. Improved flame retardancy by synergy between cyclotetrasiloxane and phosphaphenanthrene/triazine compounds in epoxy thermoset. Polym. Int. 2017, 66, 1883–1890. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B. The effect of different impact modifiers in halogen-free flame retarded polycarbonate blends—II. Fire behavior. Polym. Degrad. Stab. 2009, 94, 2204–2212. [Google Scholar] [CrossRef]
- Tang, S.; Qian, L.J.; Qiu, Y.; Dong, Y.P. Synergistic flame-retardant effect and mechanisms of boron/phosphorus compounds on epoxy resins. Polym. Adv. Technol. 2018, 29, 641–648. [Google Scholar] [CrossRef]
- Zhan, Z.S.; Xu, M.J.; Li, B. Synergistic effects of sepiolite on the flame retardant properties and thermal degradation behaviors of polyamide 66/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar] [CrossRef]
- Le Bras, M.; Bourbigot, S.; Félix, E.; Pouille, F.; Siat, C.; Traisnel, M. Characterization of a polyamide-6-based intumescent additive for thermoplastic formulations. Polymer 2000, 41, 5283–5296. [Google Scholar] [CrossRef]
- Ishida, H.; Wellinghoff, S.T.; Baer, E.; Koenig, J.L. Spectroscopic studies of poly[N,N′-bis(phenoxyphenyl)pyromellitimide]. 1. Structures of the polyimide and three model compounds. Macromolecules 1980, 13, 826–834. [Google Scholar] [CrossRef]
- Mckittrick, P.T.; Katon, J.E. Infrared and raman group frequencies of cyclic imides. Appl. Spectrosc. 1990, 44, 812–817. [Google Scholar] [CrossRef]
- Basset, F.; Lefrant, A.; Pascal, T.; Gallot, B.; Sillion, B. Crystalline polyimide particles generated via thermal imidization in a heterogeneous medium. Polym. Adv. Technol. 1998, 9, 202–209. [Google Scholar] [CrossRef]
- Schartel, B.; Bartholmai, M.; Knoll, U. Some comments on the use of cone calorimeter data. Polym. Degrad. Stab. 2005, 88, 540–547. [Google Scholar] [CrossRef]
- Qiu, Y.; Qian, L.J.; Feng, H.S.; Jin, S.L.; Hao, J.W. Toughening effect and flame-retardant behaviors of phosphaphenanthrene/phenylsiloxane bigroup macromolecules in epoxy thermoset. Macromolecules 2018, 51, 9992–10002. [Google Scholar] [CrossRef]







| Samples | PA6 (wt %) | GF (wt %) | ADP (wt %) | API (wt %) | Antioxidant1010 (wt %) | Antioxidant168 (wt %) |
|---|---|---|---|---|---|---|
| 1%API/11%ADP/GF/PA6 | 57.4 | 30 | 11.0 | 1.0 | 0.4 | 0.2 |
| 3%API/9%ADP/GF/PA6 | 57.4 | 30 | 9.0 | 3.0 | 0.4 | 0.2 |
| 5%API/7%ADP/GF/PA6 | 57.4 | 30 | 7.0 | 5.0 | 0.4 | 0.2 |
| 12%ADP/GF/PA6 | 57.4 | 30 | 12.0 | 0.0 | 0.4 | 0.2 |
| GF/PA6 | 69.4 | 30 | 0.0 | 0.0 | 0.4 | 0.2 |
| Samples | Td,2% (°C) | Td,max (°C) | Residue at 800 °C (%) |
|---|---|---|---|
| API | 577 | 620 | 56.2 |
| ADP | 431 | 493 | 15.3 |
| 1%API/11%ADP/GF/PA6 | 386 | 441 | 34.0 |
| 3%API/9%ADP/GF/PA6 | 391 | 441 | 35.2 |
| 5%API/7%ADP/GF/PA6 | 383 | 444 | 37.0 |
| 12%ADP/GF/PA6 | 387 | 447 | 33.0 |
| GF/PA6 | 399 | 468 | 30.4 |
| Samples | LOI (%) | UL94 | |||
|---|---|---|---|---|---|
| av-t1 (s) | av-t2 (s) | Dripping | Rating | ||
| 1%API/11%ADP/GF/PA6 | 35.4 | 1.4 | 1.7 | NO | V-0 |
| 3%API/9%ADP/GF/PA6 | 34.7 | 1.2 | 4.1 | NO | V-0 |
| 5%API/7%ADP/GF/PA6 | 34.2 | 2.2 | 4.8 | NO | V-0 |
| 12%ADP/GF/PA6 | 34.0 | 1.2 | 2.1 | NO | V-0 |
| GF/PA6 | 23.1 | 34.2 | -- | YES | No rating |
| Samples | TTI (s) | THR (MJ/m2) | pk-HRR (kW/m2) | Residue (wt %) | av-EHC (MJ/kg) | av-COY (kg/kg) | av-CO2Y (kg/kg) | TSR (m2/m2) |
|---|---|---|---|---|---|---|---|---|
| 1%API/11%ADP/GF/PA6 | 57 ± 1 | 81.9 ± 1.3 | 667 ± 18 | 31.5 ± 0.3 | 28.8 ± 0.4 | 0.15 ± 0.01 | 2.12 ± 0.18 | 1858 ± 49 |
| 3%API/9%ADP/GF/PA6 | 46 ± 1 | 81.8 ± 0.5 | 554 ± 22 | 32.5 ± 0.3 | 28.4 ± 0.5 | 0.15 ± 0.02 | 2.13 ± 0.22 | 1872 ± 70 |
| 5%API/7%ADP/GF/PA6 | 44 ± 1 | 80.6 ± 2.1 | 497 ± 15 | 36.1 ± 0.4 | 28.2 ± 0.4 | 0.14 ± 0.01 | 2.17 ± 0.13 | 1821 ± 54 |
| 12%ADP/GF/PA6 | 42 ± 1 | 84.3 ± 0.8 | 604 ± 21 | 31.0 ± 0.5 | 29.6 ± 0.6 | 0.15 ± 0.02 | 2.19 ± 0.20 | 1889 ± 40 |
| GF/PA6 | 33 ± 2 | 116.5 ± 1.7 | 817 ± 36 | 30.1 ± 0.4 | 36.4 ± 0.9 | 0.03 ± 0.01 | 2.84 ± 0.28 | 739 ± 68 |
| Samples | Tensile Strength (MPa) | Impact Strength (kJ/m2) | Tensile Strain at Break (%) |
|---|---|---|---|
| 1%API/11%ADP/GF/PA6 | 71.5 ± 1.9 | 27.0 ± 1.1 | 3.7 ± 0.3 |
| 3%API/9%ADP/GF/PA6 | 78.8 ± 0.6 | 27.2 ± 1.5 | 3.8 ± 0.4 |
| 5%API/7%ADP/GF/PA6 | 83.5 ± 0.6 | 28.5 ± 1.5 | 4.3 ± 0.4 |
| 12%ADP/GF/PA6 | 70.0 ± 3.4 | 20.6 ± 1.2 | 2.5 ± 0.4 |
| GF/PA6 | 84.4 ± 1.4 | 27.3 ±2.2 | 2.8 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Qiu, Y.; Qian, L.; Chen, Y.; Xu, B.; Xin, F. Flame Inhibition and Charring Effect of Aromatic Polyimide and Aluminum Diethylphosphinate in Polyamide 6. Polymers 2019, 11, 74. https://doi.org/10.3390/polym11010074
Feng H, Qiu Y, Qian L, Chen Y, Xu B, Xin F. Flame Inhibition and Charring Effect of Aromatic Polyimide and Aluminum Diethylphosphinate in Polyamide 6. Polymers. 2019; 11(1):74. https://doi.org/10.3390/polym11010074
Chicago/Turabian StyleFeng, Haisheng, Yong Qiu, Lijun Qian, Yajun Chen, Bo Xu, and Fei Xin. 2019. "Flame Inhibition and Charring Effect of Aromatic Polyimide and Aluminum Diethylphosphinate in Polyamide 6" Polymers 11, no. 1: 74. https://doi.org/10.3390/polym11010074
APA StyleFeng, H., Qiu, Y., Qian, L., Chen, Y., Xu, B., & Xin, F. (2019). Flame Inhibition and Charring Effect of Aromatic Polyimide and Aluminum Diethylphosphinate in Polyamide 6. Polymers, 11(1), 74. https://doi.org/10.3390/polym11010074