Selective Localization of Carbon Black in Bio-Based Poly (Lactic Acid)/Recycled High-Density Polyethylene Co-Continuous Blends to Design Electrical Conductive Composites with a Low Percolation Threshold
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Preparation of PLA/HDPE/CB Electrically Conductive Composites
2.3. Characterization
3. Results and Discussion
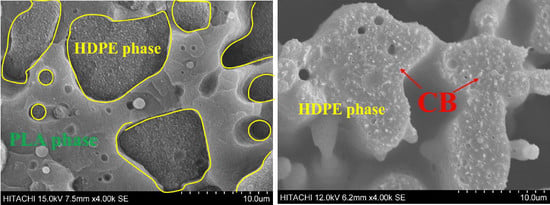
3.1. Morphology of PLA/HDPE Blends
3.2. Effect of CB on the Morphology of PLA/HDPE Blends
3.3. Selective Distribution of CB Particles
3.4. Electrical Conductivity
3.5. Thermal Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zolali, A.M.; Favis, B.D. Toughening of Cocontinuous Polylactide/Polyethylene Blends via an Interfacially Percolated Intermediate Phase. Macromolecules 2018, 51, 3572–3581. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, J.; Yang, X.; Xiao, P. Morphology and properties of biodegradable poly (lactic acid)/poly (butylene adipate-co-terephthalate) blends with different viscosity ratio. Polym. Test 2017, 60, 58–67. [Google Scholar]
- Barlow, J.; Paul, D. Mechanical Compatibilization of Immiscible Blends. Polym. Eng. Sci. 1984, 24, 525–534. [Google Scholar] [CrossRef]
- Zhang, C.L.; Feng, L.F.; Gu, X.P.; Hoppe, S.; Hu, G.H. Blend composition dependence of the compatibilizing efficiency of graft copolymers for immiscible polymer blends. Polym. Eng. Sci. 2010, 50, 2243–2251. [Google Scholar] [CrossRef]
- Hu, G.H.; Lambla, M. Chemical reactions between immiscible polymers in the melt: Transesterification of poly(ethylene-co-methyl acrylate) with mono-hydroxylated polystyrenes. J. Polym. Sci. Pol. Chem. 1995, 33, 97–107. [Google Scholar] [CrossRef]
- Zolali, A.M.; Favis, B.D. Compatibilization and toughening of co-continuous ternary blends via partially wet droplets at the interface. Polymers 2017, 114, 277–288. [Google Scholar] [CrossRef]
- Gao, T.; Li, Y.Y.; Bao, R.Y.; Liu, Z.Y.; Xie, B.H.; Yang, M.B.; Yang, W. Tailoring co-continuous like morphology in blends with highly asymmetric composition by MWCNTs: Towards biodegradable high-performance electrical conductive poly(l-lactide)/poly(3-hydroxybutyrate-co-4-hydroxybutyrate) blends. Compos. Sci. Technol. 2017, 152, 111–119. [Google Scholar] [CrossRef]
- Bai, L.; Sharma, R.; Cheng, X.; Macosko, C.W. Kinetic Control of Graphene Localization in Co-continuous Polymer Blends via Melt Compounding. Langmuir 2018, 34, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, X.; Li, Y. Formation of co-continuous PLLA/PC blends with significantly improved physical properties by reactive comb polymers. J. Appl. Polym. Sci. 2018, 135, 46047. [Google Scholar]
- Cao, W.T.; Chen, F.F.; Zhu, Y.J.; Zhang, Y.G.; Jiang, Y.Y.; Ma, M.G.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 12, 4583–4593. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, S.; Wang, F.; Wen, B.; Han, C.; Ding, Y.; Yang, M. Graphene networks with low percolation threshold in ABS nanocomposites: selective localization and electrical and rheological properties. ACS Appl. Mater. Interfaces 2014, 6, 12252–12260. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Zhang, B.B.; Hong, R.Y.; Kumar, M.R.; Xie, C.R. Enhanced electrical conductivity and mechanical properties of ABS/EPDM composites filled with graphene. Compos. Part B-Eng. 2015, 83, 66–74. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, H.; Luo, Y.; Zhang, Q.; Fu, Q. Achieving a low electrical percolation threshold and superior mechanical performance in poly(l-lactide)/thermoplastic polyurethane/carbon nanotubes composites via tailoring phase morphology with the aid of stereocomplex crystallites. RSC Adv. 2017, 7, 11076–11084. [Google Scholar] [CrossRef]
- Gubbels, F.; JbrOme, R.; Teyssib, P.; Vanlathem, E.; Deltour, R.; Calderone, A.; Parentb, V.; Brbdas, J.L. Selective Localization of Carbon Black in Immiscible Polymer Blends: A Useful Tool to Design Electrical Conductive Composites. Macromolecules 1994, 27, 1972–1974. [Google Scholar] [CrossRef]
- Qi, X.; Dong, P.; Liu, Z.; Liu, T.; Fu, Q. Selective localization of multi-walled carbon nanotubes in bi-component biodegradable polyester blend for rapid electroactive shape memory performance. Compos. Sci. Technol. 2016, 125, 38–46. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Li, X.; Xu, Y.; Jiang, J.; Hou, J.; Li, Q.; Turng, L.S. Selective localization of graphene oxide in electrospun polylactic acid/poly(ε-caprolactone) blended nanofibers. Polym. Test 2017, 59, 396–403. [Google Scholar] [CrossRef]
- Wu, D.; Lin, D.; Zhang, J.; Zhou, W.; Zhang, M.; Zhang, Y.; Wang, D.; Lin, B. Selective Localization of Nanofillers: Effect on Morphology and Crystallization of PLA/PCL Blends. Macromol. Chem. Phys. 2011, 212, 613–626. [Google Scholar] [CrossRef]
- Wu, D.; Sun, Y.; Lin, D.; Zhou, W.; Zhang, M.; Yuan, L. Selective Localization Behavior of Carbon Nanotubes: Effect on Transesterification of Immiscible Polyester Blends. Macromol. Chem. Phys. 2011, 212, 1700–1709. [Google Scholar] [CrossRef]
- Goldel, A.; Kasaliwal, G.; Potschke, P. Selective Localization and Migration of Multiwalled Carbon Nanotubes in Blends of Polycarbonate and Poly(styrene-acrylonitrile). Macromol. Rapid Commun. 2009, 30, 423–429. [Google Scholar] [CrossRef]
- Huang, J.; Mao, C.; Zhu, Y.; Jiang, W.; Yang, X. Control of carbon nanotubes at the interface of a co-continuous immiscible polymer blend to fabricate conductive composites with ultralow percolation thresholds. Carbon 2014, 73, 267–274. [Google Scholar] [CrossRef]
- Arias, V.; Odelius, K.; Hoglund, A.; Albertsson, A.C. Homocomposites of Polylactide (PLA) with Induced Interfacial Stereocomplex Crystallites. ACS Sustain. Chem. Eng. 2015, 3, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lu, X. Morphology and properties of super-toughened bio-based poly(lactic acid)/poly(ethylene-co-vinyl acetate) blends by peroxide-induced dynamic vulcanization and interfacial compatibilization. Polym. Test 2016, 56, 354–363. [Google Scholar]
- Zare, Y.; Garmabi, H.; Rhee, K.Y. Structural and phase separation characterization of poly(lactic acid)/poly(ethylene oxide)/carbon nanotube nanocomposites by rheological examinations. Compos. Part B-Eng. 2018, 144, 1–10. [Google Scholar] [CrossRef]
- Fu, Q.; Men, Y.; Strobl, G. Understanding of the tensile deformation in HDPE/LDPE blends based on their crystal structure and phase morphology. Polymers 2003, 44, 1927–1933. [Google Scholar] [CrossRef]
- Swain, S.K.; Isayev, A.I. Effect of ultrasound on HDPE/clay nanocomposites: Rheology, structure and properties. Polymers 2007, 48, 281–289. [Google Scholar] [CrossRef]
- Lu, X.; Tang, L.; Wang, L.; Zhao, J.; Li, D.; Wu, Z.; Xiao, P. Morphology and properties of bio-based poly (lactic acid)/high-density polyethylene blends and their glass fiber reinforced composites. Polym. Test 2016, 54, 90–97. [Google Scholar]
- Bittmann, B.; Bouza, R.; Barral, L.; Castro-Lopez, M.; Dopico-Garcia, S. Morphology and thermal behavior of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(butylene adipate-co-terephthalate)/clay nanocomposites. Polym. Compos. 2015, 36, 2051–2058. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Yuan, D.; Xu, C.; Cao, L.; Liang, X. Bio-Based PLA/NR-PMMA/NR Ternary Thermoplastic Vulcanizates with Balanced Stiffness and Toughness: “Soft–Hard” Core–Shell Continuous Rubber Phase, In Situ Compatibilization, and Properties. ACS Sustain. Chem. Eng. 2018, 6, 6488–6496. [Google Scholar] [CrossRef]
- Doğan, F.; Şirin, K.; Kaya, I.S.; Balcan, M. The influence of CaCO3 filler component on thermal decomposition process of PP/LDPE/DAP ternary blend. Polym. Adv. Technol. 2010, 21, 512–519. [Google Scholar]
- Liu, W.; Yang, Y.; Nie, M. Constructing a double-percolated conductive network in a carbon nanotube/polymer-based flexible semiconducting composite. Compos. Sci. Technol. 2018, 154, 45–52. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, H.; Cao, X.; Zhao, S.; Dai, K.; Yan, X.; Zheng, G.; Liu, C.; Shen, C.; Guo, Z. Electrically conductive thermoplastic polyurethane/polypropylene nanocomposites with selectively distributed graphene. Polymers 2016, 97, 11–19. [Google Scholar] [CrossRef]
- Fowkes, F.M. Determination of interfacial tensions, contact angles, and dispersion forces in surfaces by assuming additivity of intermolecular interactions in surfaces. J. Phys. Chem. A 1962, 66, 382. [Google Scholar] [CrossRef]
- Wendt, D.O.R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar]
- Dalal, E. Calculation of solid surface tensions. Langmuir 2002, 3, 1009–1015. [Google Scholar] [CrossRef]








| Samples | (°) | (°) | (MJ/m2) | (MJ/m2) | (MJ/m2) |
|---|---|---|---|---|---|
| CB | 89.11.0 | 45.81.1 | 35.3 | 1.6 | 36.9 |
| HDPE | 87.91.2 | 56.21.0 | 28.5 | 2.1 | 30.6 |
| PLA | 84.20.9 | 68.50.8 | 16.5 | 8.7 | 25.2 |
| CB Content | (°C) | (°C) | (°C) | (°C) | (J/g) | (J/g) | (J/g) | (%) | (%) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 70.6 | 168.3 | 136.6 | 117.6 | 10.2 | 29.1 | 59.4 | 29.0 | 67.8 |
| 5wt % | 70.7 | 169.1 | 135.9 | 117.5 | 5.8 | 27.3 | 58.7 | 34.8 | 70.5 |
| 10wt % | 70.5 | 168.7 | 136.5 | 116.8 | 2.3 | 26.2 | 57.5 | 40.7 | 72.9 |
| CB Content | (°C) | (°C) | (°C) | Charred Residues at 600 °C (wt %) |
|---|---|---|---|---|
| 0 | 322.7 | 362.2 | 465.3 | 0.3 |
| 5wt % | 332.2 | 363.5 | 471.7 | 5.2 |
| 10wt % | 339.1 | 362.9 | 478.1 | 9.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Kang, B.; Shi, S. Selective Localization of Carbon Black in Bio-Based Poly (Lactic Acid)/Recycled High-Density Polyethylene Co-Continuous Blends to Design Electrical Conductive Composites with a Low Percolation Threshold. Polymers 2019, 11, 1583. https://doi.org/10.3390/polym11101583
Lu X, Kang B, Shi S. Selective Localization of Carbon Black in Bio-Based Poly (Lactic Acid)/Recycled High-Density Polyethylene Co-Continuous Blends to Design Electrical Conductive Composites with a Low Percolation Threshold. Polymers. 2019; 11(10):1583. https://doi.org/10.3390/polym11101583
Chicago/Turabian StyleLu, Xiang, Benhao Kang, and Shengyu Shi. 2019. "Selective Localization of Carbon Black in Bio-Based Poly (Lactic Acid)/Recycled High-Density Polyethylene Co-Continuous Blends to Design Electrical Conductive Composites with a Low Percolation Threshold" Polymers 11, no. 10: 1583. https://doi.org/10.3390/polym11101583
APA StyleLu, X., Kang, B., & Shi, S. (2019). Selective Localization of Carbon Black in Bio-Based Poly (Lactic Acid)/Recycled High-Density Polyethylene Co-Continuous Blends to Design Electrical Conductive Composites with a Low Percolation Threshold. Polymers, 11(10), 1583. https://doi.org/10.3390/polym11101583