Adsorption of Pb2+ from Aqueous Solutions Using Novel Functionalized Corncobs via Atom Transfer Radical Polymerization
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Standards
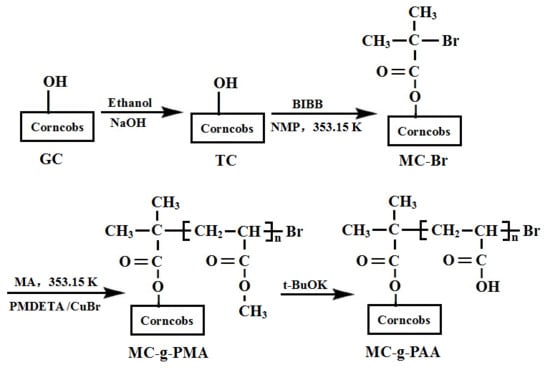
2.2. Preparation of the Corncobs
2.3. Synthesis
2.3.1. Immobilization of ATRP Initiators on the Corncobs
2.3.2. Grafting of Poly(Methyl Acrylate) on the Corncobs
2.3.3. Preparation of Functionalized Corncobs
2.4. Characterization
2.5. Pb2+ Solutions
2.6. Adsorption Experiments
3. Results and Discussion
3.1. Synthesis
3.2. Characterization
3.3. Effect of Solid/Liquid Ratio
3.4. Effect of Working Solution pH
3.5. Adsorption Thermodynamics
3.6. Adsorption Isotherms
3.7. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Almasian, A.; Giyahi, M.; Chizari Fard, G.; Dehdast, S.A.; Maleknia, L. Removal of Heavy Metal Ions by Modified PAN/PANI-Nylon Core-Shell Nanofibers Membrane: Filtration Performance, Antifouling and Regeneration Behavior. Chem. Eng. J. 2018, 351, 1166–1178. [Google Scholar] [CrossRef]
- Vimalnath, S.; Subramanian, S. Studies on the biosorption of Pb(II) ions using Pseudomonas putida. Sep. Sci. Technol. 2018, 53, 2550–2562. [Google Scholar] [CrossRef]
- Qu, J.; Meng, X.; You, H.; Ye, X.; Du, Z. Utilization of rice husks functionalized with xanthates as cost-effective biosorbents for optimal Cd(II) removal from aqueous solution via response surface methodology. Bioresour. Technol. 2017, 241, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yuan, S.; Lv, L.; Tan, G.; Liang, B.; Pehkonen, S.O. Poly (methacrylic acid)-grafted chitosan microspheres via surface-initiated ATRP for enhanced removal of Cd(II) ions from aqueous solution. J. Coll. Interf. Sci. 2013, 405, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zidelmal, N.; Aubry-Barroca, N.; Lepoittevin, B.; Mellah, M.; Costa, L.; Ozanam, F.; Gouget-Laemmel, A.C.; Schulz, E.; Roger, P. Synthesis, characterization and catalytic properties of salen-containing polymers obtained by atom transfer radical polymerization. Polymer 2018, 135, 261–270. [Google Scholar] [CrossRef]
- Kumar, S.; Karfa, P.; Madhuri, R.; Sharma, P.K. Designing of fluorescent and magnetic imprinted polymer for rapid, selective and sensitive detection of imidacloprid via activators regenerated by the electron transfer-atom transfer radical polymerization (ARGET-ATRP) technique. J. Phys. Chem. Solids 2018, 116, 222–233. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Yang, Q.; Huang, L.; Chen, L.; Ni, Y.; Xiao, H. Temperature and pH Responsive Cellulose Filament/Poly (NIPAM-co-AAc) Hybrids as Novel Adsorbent towards Pb(II) Removal. Carbohydr. Polymers 2018, 195, 495–504. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, P.; Yang, Z.; Lv, L.; Tang, S.; Liang, B. Successive grafting of poly(hydroxyethyl methacrylate) brushes and melamine onto chitosan microspheres for effective Cu(II) uptake. Int. J. Biol. Macromol. 2018, 109, 287–302. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, C.; Cai, P.; Bai, N.; Xu, X. Intelligent self-healing superhydrophobic modification of cotton fabrics via surface-initiated ARGET ATRP of styrene. Chem. Eng. J. 2017, 323, 134–142. [Google Scholar] [CrossRef]
- Jia, J.; Liu, C.; Wang, L.; Liang, X.; Chai, X. Double functional polymer brush-grafted cotton fiber for the fast visual detection and efficient adsorption of cadmium ions. Chem. Eng. J. 2018, 347, 631–639. [Google Scholar] [CrossRef]
- Niu, L.; Deng, S.; Yu, G.; Huang, J. Efficient removal of Cu(II), Pb(II), Cr(VI) and As(V) from aqueous solution using an aminated resin prepared by surface-initiated atom transfer radical polymerization. Chem. Eng. J. 2010, 165, 751–757. [Google Scholar] [CrossRef]
- Kuang, S.P.; Wang, Z.Z.; Liu, J.; Wu, Z.C. Preparation of triethylene-tetramine grafted magnetic chitosan for adsorption of Pb(II) ion from aqueous solutions. J. Hazard. Mater. 2013, 260, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Q.; Pu, S.; Wang, H.; Xia, K.; Han, B.; Zhou, C. Carboxyl-functionalized lotus seedpod: A highly efficient and reusable agricultural waste-based adsorbent for removal of toxic Pb2+ ions from aqueous solution. Coll. Surf. A 2019, 568, 391–401. [Google Scholar] [CrossRef]
- Park, J.; Bae, J.; Jin, K.; Park, J. Carboxylate-functionalized organic nanocrystals for high-capacity uraniumsorbents. J. Hazard. Mater. 2019, 371, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, T.; Seo, C.W.; Marshall, W.E. Removal of Selected Metal Ions from Aqueous Solution Using Modified Corncobs. Bioresour. Technol. 2001, 78, 133–139. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Bernal-Jacome, L.A.; Acosta-Rodriguez, I. Adsorption of cadmium(II) from aqueous solution on natural and oxidized corncob. Sep. Purif. Technol. 2005, 45, 41–49. [Google Scholar] [CrossRef]
- Khan, M.N.; Wahab, M.F. Characterization of chemically modified corncobs and its application in the removal of metal ions from aqueous solution. J. Hazard. Mater. 2007, 141, 237–244. [Google Scholar] [CrossRef]
- Tan, G.; Yuan, H.; Liu, Y.; Xiao, D. Removal of lead from aqueous solution with native and chemically modified corncobs. J. Hazard. Mater. 2010, 174, 740–745. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, H.; Zhou, L.; Zhang, F. Confined polymerization: ARGET ATRP of MMA in the nanopores of modified SBA-15. Polymer 2017, 114, 180–188. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Huang, X.; Wang, W.; Sun, P.; Li, Y. Adsorption of Pb2+ from aqueous solutions using Fe-Mn binary oxides-loaded biochar: Kinetics, isotherm and thermodynamic studies. Environ. Technol. 2019, 40, 1851–1861. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, W.; Norinaga, K.; Fang, J.; Wang, Y.; Zong, Z.; Wei, X. Separation of phenols and ketones from bio-oil produced from ethanolysis of wheat stalk. Sep. Purif. Technol. 2015, 152, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Lin, H.; Li, B.; Dong, Y.; He, Y.; Wang, L. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour. Technol. 2018, 259, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhao, Y.; Qiu, P.; Lin, D.; Qian, J.; Hou, H.; Pei, J. Investigation of the relationship between infrared structure and pyrolysis reactivity of coals with different ranks. Fuel 2018, 216, 521–530. [Google Scholar] [CrossRef]
- Wen, X.; Sun, N.; Yan, C.; Zhou, S.; Pang, T. Rapid removal of Cr(VI) ions by densely grafted corn stalk fibers: High adsorption capacity and excellent recyclable property. J. Taiwan Inst. Chem. Eng. 2018, 89, 95–104. [Google Scholar] [CrossRef]
- Lin, R.C.; Mohamed, G.M.; Chen, T.; Kuo, S.W. Coumarin- and Carboxyl-Functionalized Supramolecular Polybenzoxazines Form Miscible Blends with Polyvinylpyrrolidone. Polymers 2017, 9, 146. [Google Scholar] [CrossRef]
- Thorsheim, K.; Manner, S.; Ellervik, U. Expanding the scope of methyl xanthate esters-From Barton-McCombie reaction auxiliary to versatile protective group. Tetrahedron 2017, 73, 6329–6333. [Google Scholar] [CrossRef]
- Zhu, B.; Fan, T.; Zhang, D. Adsorption of copper ions from aqueous solution by citric acid modified soybean straw. J. Hazard. Mater. 2008, 153, 300–308. [Google Scholar] [CrossRef]
- Song, Z.; Li, W.; Liu, W.; Yang, Y.; Wang, N.; Wang, H.; Gao, H. Novel magnetic lignin composite sorbent for chromium(VI) adsorption. RSC Adv. 2015, 5, 13028–13035. [Google Scholar] [CrossRef]
- Tang, N.; Niu, C.G.; Li, X.T.; Liang, C.; Guo, H.; Lin, L.S.; Zheng, C.W.; Zeng, G.M. Efficient removal of Cd2+ and Pb2+ from aqueous solution with amino- and thiol-functionalized activated carbon: Isotherm and kinetics modeling. Sci. Total Environ. 2018, 635, 1331–1344. [Google Scholar] [CrossRef]
- Bamidele, J.O.; Patience, M.S.; Gabriel, O.A.; Johannes, S.M. Removal of Pb2+ from Water by Synthesized Tannin Resins from Invasive South African Trees. Water 2018, 10, 648–661. [Google Scholar]
- Gülen, J.; Zorbay, F. Methylene Blue Adsorption on a Low Cost Adsorbent-Carbonized Peanut Shell. Water Environ. Res. 2017, 89, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Chukwuemeka-Okorie, H.O.; Ekemezie, P.N.; Akpomie, K.G.; Olikagu, C.S. Calcined Corncob-Kaolinite Combo as New Sorbent for Sequestration of Toxic Metal Ions from Polluted Aqua Media and Desorption. Front. Chem. 2018, 6, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, A.; Vidhyadevi, T.; Kalaivani, S.S.; Baskaralingam, P.; Anuradha, C.D.; Sivanesan, S. Kinetic studies and isotherm modeling for the removal of Ni2+ and Pb2+ ions by modified activated carbon using sulfuric acid. Environmental Progress & Sustainable Energy 2014, 33, 844–854. [Google Scholar]
- Wang, X.; Li, X.; Liu, G.; He, Y.; Chen, C.; Liu, X.; Li, G.; Gu, Y.; Zhao, Y. Mixed heavy metals removal from wastewater by discarded mushroom-stick biochar: Adsorption properties and mechanisms. Environ. Sci. Proces. Impacts 2019, 21, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Jiang, X.J.; Jia, L.; Ai, T.; Wu, H. Kinetic and thermodynamic studies on adsorption of Cu2+, Pb2+, methylene blue and malachite green from aqueous solution using AMPS-modified hazelnut shell powder. Chem. Res. Chin. Univ. 2017, 33, 112–118. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rehman, W.; Waseem, M.; Haq, S.; Shah, K.H.; Kang, P. Adsorption of Pb2+ ions on novel ternary nanocomposite of tin, iron and titania. Mater. Res. Express 2018, 7, 189–197. [Google Scholar] [CrossRef]
- Liu, S.; Duan, Z.; He, C.; Xu, X.; Li, T.; Li, Y.; Li, X.; Wang, Y.; Xu, L. Rapid removal of Pb2+ from aqueous solution by phosphate-modified baker’s yeast. RSC Adv. 2018, 8, 8026–8038. [Google Scholar] [CrossRef]
- Narada, B.D.; Fowler, R.E.; Pittman, C.U.; Dinesh, M.; Todd, M. Lead (Pb2+) sorptive removal using chitosan-modified biochar: Batch and fixed-bed studies. RSC Adv. 2018, 8, 25368–25377. [Google Scholar]
- Chen, Z.; Zhang, J.; Huang, L. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Integr. Agric. 2019, 18, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, S.; Wang, T.; Lei, Z.; Zhu, M.; Dai, X.; Liu, F.; Li, J.; Yin, H. Structure and properties of vanadium-doped α-MnO2 and enhanced Pb2+ adsorption phenol/photocatalytic degradation. Mater. Chem. Phys. 2018, 208, 258–267. [Google Scholar] [CrossRef]
- Farhan, S.N.; Khadom, A.A. Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int. J. Ind. Chem. 2015, 6, 119–130. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhang, Z.; Awasthi, M.K.; Du, D.; Dang, P.; Huang, Q.; Zhang, Y.; Wang, L. Recovery of phosphate and dissolved organic matter from aqueous solution using a novel CaO-MgO hybrid carbon composite and its feasibility in phosphorus recycling. Sci. Total Environ. 2018, 642, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Takdastan, A.; Samarbaf, S.; Tahmasebi, Y.; Alavi, N.; Babaei, A.A. Alkali modified oak waste residues as a cost-effective adsorbent for enhanced removal of cadmium from water: Isotherm, kinetic, thermodynamic and artificial neural network modeling. J. Ind. Eng. Chem. 2019, 78, 352–363. [Google Scholar] [CrossRef]
- José, A.P.; María, I.R.; María, I.F.; Carmen, L.; María, T.M.; Santiago, L.; Vicente, M.G.; Paola, S.; Pinalysa, C.; Paola, F.; et al. Adsorption Properties of β- and Hydroxypropyl-β-Cyclodextrins Cross-Linked with Epichlorohydrin in Aqueous Solution. A Sustainable Recycling Strategy in Textile Dyeing Process. Polymers 2019, 11, 252–272. [Google Scholar]
- Salam, M.A.; Al-Zhrani, G.; Kosa, S.A. Removal of heavy metal ions from aqueous solution by multi-walled carbon nanotubes modified with 8-hydroxyquinoline: Kinetic study. J. Ind. Eng. Chem. 2014, 20, 572–580. [Google Scholar] [CrossRef]
















| T (K) | (L/g) | (kJ/mol) | (J/mol/K) | (kJ/mol) |
|---|---|---|---|---|
| 303.15 | 2.115 | −1.888 | 15.86 | 2.926 |
| 313.15 | 2.197 | −2.050 | ||
| 323.15 | 2.238 | −2.164 | ||
| 333.15 | 2.352 | −2.369 | ||
| 343.15 | 2.438 | −2.543 | ||
| 353.15 | 2.476 | −2.662 |
| Model | Parameter | Value |
|---|---|---|
| Langmuir | (mg/g) | 342.47 |
| (min−1) | 0.3023 | |
| 0.0074–0.0698 | ||
| R2 | 0.9994 | |
| Freundlich | (mg/g) | 124.95 |
| 4.97 | ||
| R2 | 0.8528 | |
| Temkin | (mg/L) | 27.90 |
| (J/mol) | 0.0160 | |
| R2 | 0.9317 | |
| Dubinin–Radushkevich | (mg/g) | 313.97 |
| (kJ/mol) | 1346 | |
| R2 | 0.9643 | |
| Harkins–Jura | 27778 | |
| 2.306 | ||
| R2 | 0.6313 |
| Raw Material | Adsorbent | References | |
|---|---|---|---|
| Corncobs | MC-g-PAA | 342.47 | Present study |
| Pristine corncobs | 16.22 | [18] | |
| Hydrolyzed corncobs | 43.41 | ||
| Esterified corncobs | 7.89 | ||
| Corn cob-kaolinite clay | 23.26 | [32] | |
| Acid-modified Activated Carbon (Corncob) | 29.47 | [33] | |
| Other materials | Discarded mushroom-stick biochar (DMB8) | 21.0 | [34] |
| AMPS-modified hazelnut shell powder | 32.74 | [35] | |
| Ternary nanocomposite (TNC) | 79.56 | [36] | |
| Phosphate-modified baker’s yeast (PMBY) | 92 | [37] | |
| Fe-Mn binary oxides-loaded biochar (BFM) | 113.72 | [20] | |
| Chitosan-Modified fast pyrolysis BioChar (CMBC) | 134 | [38] | |
| Non-Modified BioChar (NMBC) | 48.2 | ||
| Biochar(BC) made from dairy manure | 175.53 | [39] | |
| Silver wattle tannin resins | 189.30 | [30] | |
| Black wattle tannin resins | 105.70 | ||
| Green wattle tannin resins | 98.82 | ||
| Vanadium (V)-doped materials | 194.15–237.45 | [40] |
| Model | Parameter | Value |
|---|---|---|
| Experimental data | (mg/g) | 323.65 |
| First-order | (mg/g) | 320.51 |
| (min−1) | 0.4808 | |
| R2 | 0.90174 | |
| Second-order | (mg/g) | 324.68 |
| (g/mg/min) | 0.00561 | |
| (mg/g/min) | 591.72 | |
| R2 | 0.99999 | |
| Intra-particle diffusion | (mg/g/min0.5) | 5.121 |
| 272.7 | ||
| R2 | 0.4577 | |
| Elovich | α | 3.41 × 107 |
| β | 0.0578 | |
| R2 | 0.7508 | |
| Avrami-fractional | 0.4713 | |
| 1.020 | ||
| R2 | 0.9142 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhao, W. Adsorption of Pb2+ from Aqueous Solutions Using Novel Functionalized Corncobs via Atom Transfer Radical Polymerization. Polymers 2019, 11, 1715. https://doi.org/10.3390/polym11101715
Chen S, Zhao W. Adsorption of Pb2+ from Aqueous Solutions Using Novel Functionalized Corncobs via Atom Transfer Radical Polymerization. Polymers. 2019; 11(10):1715. https://doi.org/10.3390/polym11101715
Chicago/Turabian StyleChen, Shanglong, and Wei Zhao. 2019. "Adsorption of Pb2+ from Aqueous Solutions Using Novel Functionalized Corncobs via Atom Transfer Radical Polymerization" Polymers 11, no. 10: 1715. https://doi.org/10.3390/polym11101715
APA StyleChen, S., & Zhao, W. (2019). Adsorption of Pb2+ from Aqueous Solutions Using Novel Functionalized Corncobs via Atom Transfer Radical Polymerization. Polymers, 11(10), 1715. https://doi.org/10.3390/polym11101715