Bioactive Edible Films Based on Arrowroot Starch Incorporated with Cranberry Powder: Microstructure, Thermal Properties, Ascorbic Acid Content and Sensory Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Film Preparation
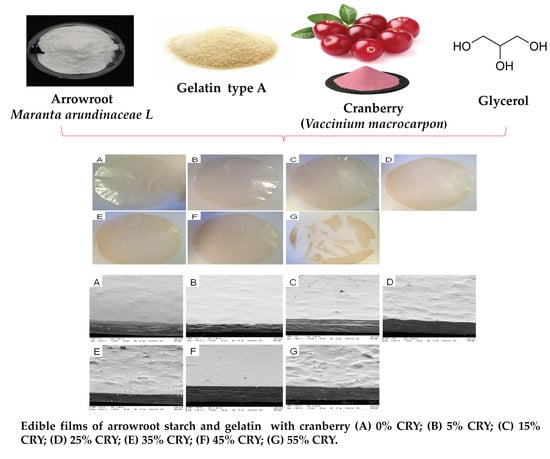
2.2.2. Visual Aspect and Microstructure
2.2.3. X-ray Diffractometry (XRD)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Determination of Ascorbic Acid Content
2.2.6. Sensory Analysis
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Visual Aspects and Microstructure
3.2. X-ray Diffractometry (XRD)
3.3. Differential Scanning Calorimetry (DSC)
3.4. Ascorbic Acid Content in Films
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Chang-Bravo, L.; López-Córdoba, A.; Martino, M. Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React. Funct. Polym. 2014, 85, 11–19. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Bertan, L.C. Development and Charaterization of Composite Films Based on Gelatin, Fatty Acids and Breu. Master’s Thesis, State University of Campinas, Sao Paulo, Brazil, 2008. [Google Scholar]
- Mohee, R.; Unmar, G.D.; Mudhoo, A.; Khadoo, P. Biodegradability of biodegradable/degradable plastic materials under aerobic and anaerobic conditions. Waste Manag. 2008, 28, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Sartori, T.; Menegalli, F.C. Development and characterization of unripe banana starch films incorporated with solid lipid microparticles containing ascorbic acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Villas-Boas, F.; Franco, C.M.L. Effect of bacterial β-amylase and fungal α-amylase on the digestibility and structural characteristics of potato and arrowroot starches. Food Hydrocoll. 2016, 52, 795–803. [Google Scholar] [CrossRef]
- Moorthy, S.N. Physicochemical and functional properties of tropical tuber starches: A review. Starch 2002, 54, 559–592. [Google Scholar] [CrossRef]
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Poppe, J. Gelatin. In Thickening and Gelling Agents for Food; Imeson, A., Ed.; Aspen Publishers: New York, NY, USA, 1987; pp. 144–168. [Google Scholar] [CrossRef]
- Kester, J.J.; Fennema, O.R. Edible biofilms and coatings: A review. Food Technol. 1986, 40, 47–59. [Google Scholar]
- Fakhouri, F.M.; Martelli, S.M.; Bertan, L.C.; Yamashita, F.; Mei, I.L.H.; Queiroz, F.P.C. Edible films made from blends of manioc starch and gelatin—Influence of different types of plasticizer and different levels of macromolecules on their properties. LWT Food Sci. Technol. 2012, 49, 149–154. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Morrugares-Carmona, R.; Wellner, N.; Cross, K.; Bajka, B.; Waldron, K.W. Development of pectin films with pomegranate juice and citric acid. Food Chem. 2016, 198, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Farias, M.G.; Fakhouri, F.M.; Carvalho, C.W.P.; Ascheri, J.L.R. Caracterização físico-química de filmes comestíveis de amido adicionado de acerola (Malphigia emarginata D.C.). Quím Nova 2012, 35, 546–552. [Google Scholar] [CrossRef]
- Cerruti, P.; Santagata, G.; Gomez d’Ayala, G.; Ambrogi, V.; Carfagna, C.; Malinconico, M.; Persico, P. Effect of a natural polyphenolic extract on the properties of a biodegradable starch-based polymer. Polym. Degrad. Stabil. 2011, 96, 839–846. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.-T. Review: Fortification of dietary biopolymers-based packaging material with bioactive plant extracts. Food Res. Int. 2012, 49, 80–91. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef] [PubMed]
- Hisano, M.; Bruschini, H.; Nicodemo, A.C.; Srougii, M. Review: Cranberries and lower urinary tract infection prevention. Clinics 2012, 67, 661–667. [Google Scholar] [CrossRef]
- Tulio, A.Z., Jr.; Jablonski, J.E.; Jackson, L.S.; Chang, C.; Edirisinghe, I.; Burton-Freeman, B. Phenolic composition, antioxidant properties, and endothelial cell function of red and white cranberry fruits. Food Chem. 2014, 157, 540–552. [Google Scholar] [CrossRef]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Nowack, R.; Schmitt, W. Cranberry juice for prophylaxis of urinary tract infections—Conclusions from clinical experience and research. Phytomedicine 2008, 15, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.F.; Wu, X.Z.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.Y.; Lu, Y.R.; Howell, A.B.; Vorsa, N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Burger, O.; Ofek, I.; Tabak, M.; Weiss, E.I.; Sharon, N.; Neeman, I. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol. Med. Microbiol. 2000, 29, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.H.; Qiu, X.J.; Bushway, A.; Harper, L. Antibacterial effects of American cranberry (Vaccinium macrocarpon) concentrate on foodborne pathogens. LWT Food Sci. Technol. 2008, 41, 1834–1841. [Google Scholar] [CrossRef]
- Lacombe, A.; Mcgivney, C.; Tadepalli, S.; Sun, X.; Wu, V.C.H. The effect of American cranberry (Vaccinium macrocarpon) constituents on the growth inhibition, membrane integrity, and injury of Escherichia coli O157:H7 and Listeria monocytogenes in comparison to Lactobacillus rhamnosus. Food Microbiol. 2013, 34, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Martinez, C.; Cuevas, F. Evaluación de la calidad culinaria y molinera del arroz. Guia de estudo; CIAT: Cali, Colombia, 1989; p. 75. [Google Scholar]
- Zavareze, E.R.; El Halal, S.L.M.; Pereira, J.M.; Radünz, A.L.; Elias, M.C.; Dias, A.R.G. Chemical characterization and extraction yield of rice starch with different amylose contents. Braz. J. Food Technol. 2009, 2, 25–30. [Google Scholar]
- Horwitz, W. Official Methods of Analysis, 3rd ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J. Sens. Stud. 2007, 4, 129–148. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Effect of incorporation of blackberry particles on the physicochemical properties of edible films of arrowroot starch. Dry Technol. 2018, 37, 1–10. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; de Oliveira, R.A. Bioactive films of arrowroot starch and blackberry pulp: Physical, mechanical and barrier properties and stability to pH and sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef]
- Shi, D.; Nannenga, B.L.; Iadanza, M.G.; Gonen, T. Three-dimensional electron cryslallography of protein microcrystals. Elife 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Dammak, I.; Lourenço, R.L.; Sobral, P.J.A. Active gelatin films incorporated with Pickering emulsions encapsulating hesperidin: Preparation and physicochemical characterization. J. Food Eng. 2019, 240, 9–20. [Google Scholar] [CrossRef]
- Jalaja, K.; Deboki, N.; Subhas, C.K.; Nirmala, R.J. Fabrication of cationized gelatin nanofibers by electrospinning for tissue regeneration. RSC Adv. 2015, 5, 89521–89530. [Google Scholar] [CrossRef]
- Otoni, C.G.; De Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.F.F.; Mattoso, L.H.C. Antimicrobial and physical mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Ivanič, F.; Jochec-Mošková, D.; Janigová, I.; Chodák, I. Physical properties of starch plasticized by a mixture of plasticizers. Eur. Polym. J. 2017, 93, 843–849. [Google Scholar] [CrossRef]
- Bergo, P.V.A.; Carvalho, R.A.; Sobral, P.J.A.; Dos Santos, R.M.C.; Da Silva, F.B.R.; Prison, J.M.; Habitante, A.M.Q.B. Physical properties of edible films based on cassava starch as affected by the plasticizer concentration. Packag. Technol. Sci. 2008, 21, 85–89. [Google Scholar] [CrossRef]
- Islam, M.Z.; Kitamura, Y.; Yamano, Y.; Kitamura, M. Effect of vacuum spray drying on the physicochemical properties, water sorption and glass transition phenomenon of orange juice powder. J. Food Eng. 2016, 169, 131–140. [Google Scholar] [CrossRef]
- Ré, M.I. Microencapsulation by spray drying. Dry Technol. 1998, 16, 1195–1236. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (spp.) by-product extract by freeze-drying. LWT-Food Sci. Technol. 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Krueger, C.G.; Moskal, K.; Fridlender, B.; Lila, M.A.; Raskin, I. Food-compatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chem. 2013, 141, 3664–3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Sample | Appearance | Colour | Flavour | Taste | Global Acceptation |
|---|---|---|---|---|---|
| 0% CRY | 7.48 ± 1.53 | 7.32 ± 1.63 | 6.46 ± 1.53 | 5.21 ± 1.74 | 6.09 ± 1.70 |
| 5% CRY | 7.29 ± 1.56 | 7.13 ± 1.62 | 6.50 ± 1.54 | 5.71 ± 1.72 | 6.25 ± 1.49 |
| 15% CRY | 7.30 ± 1.43 | 7.13 ± 1.36 | 6.58 ± 1.57 | 6.27 ± 1.57 | 6.61 ± 1.37 |
| 25% CRY | 7.38 ± 1.41 | 7.20 ± 1.51 | 6.46 ± 1.61 | 6.66 ± 1.52 | 6.77 ± 1.57 |
| 35% CRY | 7.27 ± 1.50 | 7.16 ± 1.40 | 6.60 ± 1.40 | 6.70 ± 1.64 | 6.71 ± 1.46 |
| 45% CRY | 6.73 ± 1.59 | 7.02 ± 1.41 | 6.39 ± 1.50 | 6.73 ± 1.51 | 6.63 ± 1.52 |
| 55% CRY | 6.91 ± 1.53 | 7.07 ± 1.61 | 6.60 ± 1.48 | 6.82 ± 1.57 | 6.89 ± 1.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matta Fakhouri, F.; Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I. Bioactive Edible Films Based on Arrowroot Starch Incorporated with Cranberry Powder: Microstructure, Thermal Properties, Ascorbic Acid Content and Sensory Analysis. Polymers 2019, 11, 1650. https://doi.org/10.3390/polym11101650
Matta Fakhouri F, Nogueira GF, de Oliveira RA, Velasco JI. Bioactive Edible Films Based on Arrowroot Starch Incorporated with Cranberry Powder: Microstructure, Thermal Properties, Ascorbic Acid Content and Sensory Analysis. Polymers. 2019; 11(10):1650. https://doi.org/10.3390/polym11101650
Chicago/Turabian StyleMatta Fakhouri, Farayde, Gislaine Ferreira Nogueira, Rafael Augustus de Oliveira, and José Ignacio Velasco. 2019. "Bioactive Edible Films Based on Arrowroot Starch Incorporated with Cranberry Powder: Microstructure, Thermal Properties, Ascorbic Acid Content and Sensory Analysis" Polymers 11, no. 10: 1650. https://doi.org/10.3390/polym11101650
APA StyleMatta Fakhouri, F., Nogueira, G. F., de Oliveira, R. A., & Velasco, J. I. (2019). Bioactive Edible Films Based on Arrowroot Starch Incorporated with Cranberry Powder: Microstructure, Thermal Properties, Ascorbic Acid Content and Sensory Analysis. Polymers, 11(10), 1650. https://doi.org/10.3390/polym11101650