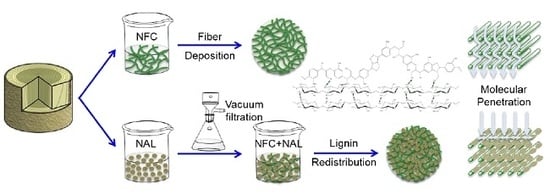
Lignin Redistribution for Enhancing Barrier Properties of Cellulose-Based Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanolized Alkali Lignin Preparation
2.3. Characterization of NAL
2.4. Lignin Redistribution and Surface Characterization
2.5. Water Contact Angle Measurement
2.6. Grease Resistance Measurement
2.7. Water Vapor Resistance Measurement
2.8. Antibacterial Activity of Deposited Paper
3. Results and Discussion
3.1. Nanocellulose Fractionation
Characterization of NAL
3.2. Surface Properties of Deposited Paper
3.3. Water Resistance of Deposited Paper
3.4. Grease Resistance of Deposited Paper
3.5. Water Vapor Resistance of Deposited Paper
3.6. Antibacterial Activity of Deposited Paper
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Azeredo, H.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Yu, J.; Ruengkajorn, K.; Crivoi, D.; Chen, C.; Buffet, J.; O’Hare, D. High gas barrier coating using non-toxic nanosheet dispersions for flexible food packaging film. Nat. Commun. 2019, 20, 2398–2405. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crop. Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends. Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Busolo, M.A.; Fernandez, P.; Ocio, M.J.; Jose, M.l. Novel silver-based nanoclay as an antimicrobial in polylactic acid food packaging coatings. Food Addit. Contam. 2010, 27, 1617–1626. [Google Scholar] [CrossRef]
- Farmahini-Farahani, M.; Xiao, H.; Zhao, Y. Poly lactic acid nanocomposites containing modified nanoclay with synergistic barrier to water vapor for coated paper. J. Appl. Polym. Sci. 2014, 131, 40952–40957. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Silvia, R.; Cristina, N. Nanoclay migration from food packaging materials. Food Addit. Contam. 2016, 33, 530–539. [Google Scholar] [CrossRef]
- Zhu, P.; Kuang, Y.; Chen, G.; Liu, Y.; Peng, C.; Hu, W. Starch/polyvinyl alcohol (PVA)-coated painting paper with exceptional organic solvent barrier properties for art preservation purposes. J. Mater. Sci. 2018, 53, 5450–5457. [Google Scholar] [CrossRef]
- Kopacic, S.; Walzl, A.; Zankel, A.; Leitner, E.; Bauer, W. Alginate and Chitosan as a Functional Barrier for Paper-Based Packaging Materials. Coatings 2018, 8, 235. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, M.; Sun, R.; Wang, X. Large scale preparation of graphene oxide/cellulose paper with improved mechanical performance and gas barrier properties by conventional papermaking method. Ind. Crop. Prod. 2016, 85, 198–203. [Google Scholar] [CrossRef]
- Herrera, M.A.; Mathew, A.P.; Oksman, K. Barrier and mechanical properties of plasticized and cross-linked nanocellulose coatings for paper packaging applications. Cellulose 2017, 24, 1–12. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose–Its barrier properties and applications in cellulosic materials: A review. Carbohyd. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Yang, Y.; Fang, G.; Dai, H. Improving cellulose nanofibrillation of waste wheat straw using the combined methods of prewashing, p-toluenesulfonic acid hydrolysis, disk grinding, and endoglucanase post-treatment. Bioresour. Technol. 2018, 256, 321–327. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A.; Mukherjee, S.; Sahu, S.; Chowdhury, S.; Mishra, M.; Talegaonkar, S.; Siddiqui, L.; Mishra, H. Wood-Based Cellulose Nanofibrils: Haemocompatibility and Impact on the Development and Behaviour of Drosophila melanogaster. Biomolecules 2019, 9, 363. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, X.; Song, P.; Wang, M.; Xu, F.; Jiang, Y.; Zhang, X. An approach for reinforcement of paper with high strength and barrier properties via coating regenerated cellulose. Carbohyd. Polym. 2018, 200, 100–105. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Du, J.; Yang, Y.; Jin, Y. Influence of lignin addition on the enzymatic digestibility of pretreated lignocellulosic biomasses. Bioresour. Technol. 2015, 181, 7–12. [Google Scholar] [CrossRef]
- Ewulonu, C.M.; Liu, X.; Wu, M.; Huang, Y. Lignin-containing cellulose nanomaterials: A promising new nanomaterial for numerous applications. J. Bioresour. Bioprod. 2019, 4, 3–10. [Google Scholar]
- Ur Rahman, O.; Shi, S.; Ding, J.; Wang, D.; Ahmad, S.; Yu, H. Lignin nanoparticles: Synthesis, characterization and corrosion protection performance. New J. Chem. 2018, 42, 3415–3425. [Google Scholar] [CrossRef]
- Wang, B.; Shi, T.; Zhang, Y.; Chen, C.; Li, Q.; Fan, Y. Lignin-based highly sensitive flexible pressure sensor for wearable electronics. J. Mater. Chem. C 2018, 6, 6423–6428. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. The self-assembly of lignin and its application in nanoparticle synthesis: A short review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef]
- Hult, E.L.; Ropponen, J.; Poppius-Levlin, K.; Ohra-Aho, T.; Tamminen, T. Enhancing the barrier properties of paper board by a novel lignin coating. Ind. Crop. Prod. 2013, 50, 694–700. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevicius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. Chem. Phys. Chem. 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- TAPPI Standard Test Method. Air Resistance of Paper (Gurley Method); T 460 om-06; TAPPI: Peachtree Corners, GA, USA, 2006. [Google Scholar]
- TAPPI Standard Test Method. Grease Resistance of Flexible Packaging Materials; T 507 cm-99; TAPPI: Peachtree Corners, GA, USA, 1999. [Google Scholar]
- TAPPI Standard Test Method. Water Vapor Transmission Rate of Sheet Materials at Normal Temperature; T 448 om-09; TAPPI: Peachtree Corners, GA, USA, 2009. [Google Scholar]
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for Identification of Plant Pathogenic Bacteria; APS Press: St. Paul, MN, USA, 1988. [Google Scholar]
- Niu, F.; Li, M.; Huang, Q.; Zhang, X.; Pan, W.; Yang, J.; Li, J. The characteristic and dispersion stability of nanocellulose produced by mixed acid hydrolysis and ultrasonic assistance. Carbohyd. Polym. 2017, 165, 197–204. [Google Scholar] [CrossRef]
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stabil. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- You, T.; Li, X.; Wang, R.; Zhang, X.; Xu, G. Effects of synergistic fungal pretreatment on structure and thermal properties of lignin from corncob. Bioresour. Technol. 2019, 272, 123–129. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Zhang, X.; Zhang, N.; Tian, D. External-field-induced gradient wetting for controllable liquid transport: From movement on the surface to penetration into the surface. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, P.; Qian, L.; Xiao, H. Fabrication of superhydrophobic paper surface via wax mixture coating. Chem. Eng. J. 2014, 250, 431–436. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, W.; He, M.; Yan, Y.; Xiao, H. Cellulase-assisted refining of bleached softwood kraft pulp for making water vapor barrier and grease-resistant paper. Cellulose 2015, 23, 891–900. [Google Scholar] [CrossRef]
- Chi, K.; Catchmark, J.M. Improved eco-friendly barrier materials based on crystalline nanocellulose/chitosan/carboxymethyl cellulose polyelectrolyte complexes. Food Hydrocoll. 2018, 80, 195–205. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chang, T.H.; Zhang, H.; Yao, C.; Zheng, Q.; Yang, V.W.; Mi, H.; Kim, M.; Cho, S.J.; Park, D.W.; et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170–7180. [Google Scholar] [CrossRef]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Gu, L.H.; Wu, W.J.; Zhao, H.F.; Jin, Y.C. Structural elucidation and antioxidant activity of lignin isolated fromrice straw and alkali oxygen black liquor. Int. J. Biol. Macromol. 2018, 116, 513–519. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J.S.; Bruni, G.; Kozanecki, M.; Kenny, J.M.; Torre, L.; Visai, L.; Puglia, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohyd. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Dai, H.; Xiao, H. Starch-Based Flexible Coating for Food Packaging Paper with Exceptional Hydrophobicity and Antimicrobial Activity. Polymers 2018, 10, 1260. [Google Scholar] [CrossRef] [Green Version]






| Samples | Duration | ||||
|---|---|---|---|---|---|
| 4 h | 8 h | 24 h | 48 h | 72 h | |
| P4 (Control) | 16.6 ± 0.6 | 30.9 ± 0.9 | 62.4 ± 0.8 | 77.0 ± 0.9 | 90.5 ± 0.8 |
| P4 (NFC-3.6 g/m2) | 0.2 ± 0.2 | 0.5 ± 0.4 | 6.6 ± 0.6 | 18.1 ± 0.8 | 37.1 ± 1.6 |
| P4 (NFC + NAL) | ND | ND | ND | ND | ND |
| P8 (Control) | 20.6 ± 0.8 | 38.9 ± 0.7 | 72.9 ± 0.9 | 92.0 ± 0.8 | 99.3 ± 0.3 |
| P8 (NFC-3.6 g/m2) | 0.3 ± 0.1 | 0.8 ± 0.6 | 6.9 ± 0.7 | 20.4 ± 0.9 | 40.8 ± 1.2 |
| P8 (NFC + NAL) | ND | ND | ND | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Guo, T.; Sun, K.; Jin, Y.; Gu, F.; Xiao, H. Lignin Redistribution for Enhancing Barrier Properties of Cellulose-Based Materials. Polymers 2019, 11, 1929. https://doi.org/10.3390/polym11121929
Wang W, Guo T, Sun K, Jin Y, Gu F, Xiao H. Lignin Redistribution for Enhancing Barrier Properties of Cellulose-Based Materials. Polymers. 2019; 11(12):1929. https://doi.org/10.3390/polym11121929
Chicago/Turabian StyleWang, Wangxia, Tianyu Guo, Kaiyong Sun, Yongcan Jin, Feng Gu, and Huining Xiao. 2019. "Lignin Redistribution for Enhancing Barrier Properties of Cellulose-Based Materials" Polymers 11, no. 12: 1929. https://doi.org/10.3390/polym11121929
APA StyleWang, W., Guo, T., Sun, K., Jin, Y., Gu, F., & Xiao, H. (2019). Lignin Redistribution for Enhancing Barrier Properties of Cellulose-Based Materials. Polymers, 11(12), 1929. https://doi.org/10.3390/polym11121929