Recent Developments about Conductive Polymer Based Composite Photocatalysts
Abstract
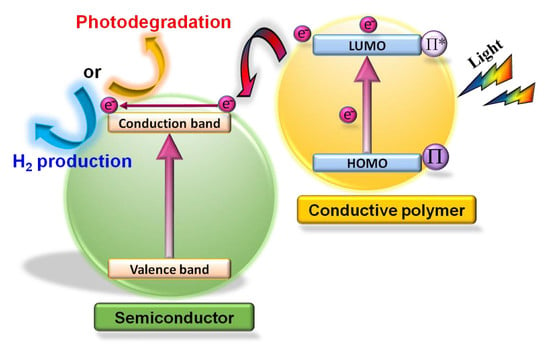
:1. Introduction
2. Polyaniline Based Photocatalysts
2.1. Photodegradation
2.1.1. Polyaniline-Bismuth Composites
2.1.2. Polyaniline-Titanium Oxide
2.1.3. Polyaniline with Other Compounds
2.2. Photocatalytic Hydrogen Production
Polyaniline with Other Compounds
3. Polypyrrole Based Photocatalysts
3.1. Photodegradation
3.1.1. Pristine Polypyrrole
3.1.2. Polypyrrole Based Composites
3.2. Photocatalytic Hydrogen Production
4. Polythiophene Based Photocatalyst
4.1. Photodegradation or Photoreduction
Polythiophene with Other Compounds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Liu, L.; Feng, Y.; Wang, L.; Bian, Z.; Li, H.; Wang, Z.L. Fluid eddy induced piezo-promoted photodegradation of organic dye pollutants in wastewater on ZnO nanorod arrays/3D Ni foam. Mater. Today 2017, 20, 501–506. [Google Scholar] [CrossRef]
- Chang, C.J.; Chao, P.Y. Efficient photocatalytic hydrogen production by doped ZnS grown on Ni foam as porous immobilized photocatalysts. Int. J. Hydrog. Energy 2018, 43, 11345–11354. [Google Scholar] [CrossRef]
- Bestetti, M.; Sacco, D.; Brunella, M.F.; Franz, S.; Amadelli, R.; Samiolo, L. Photocatalytic degradation activity of titanium dioxide sol–gel coatings on stainless steel wire meshes. Mater. Chem. Phys. 2010, 124, 1225–1231. [Google Scholar] [CrossRef]
- Hsu, M.H.; Chang, C.J. S-doped ZnO nanorods on stainless-steel wire mesh as immobilized hierarchical photocatalysts for photocatalytic H2 production. Int. J. Hydrog. Energy 2014, 39, 16524–16533. [Google Scholar] [CrossRef]
- Chang, C.J.; Huang, K.L.; Chu, K.W.; Wei, Y.H.; Tseng, I.H. Sulfonated graphene oxide-doped zincoxysulfide composites with enhanced photocatalytic hydrogen production performance. Int. J. Hydrog. Energy 2016, 41, 21755–21763. [Google Scholar] [CrossRef]
- Chang, C.J.; Wei, Y.H.; Huang, K.P. Photocatalytic hydrogen production by flower-like graphene supported ZnS composite photocatalysts. Int. J. Hydrog. Energy 2017, 42, 23578–23586. [Google Scholar] [CrossRef]
- Zou, J.; Yip, H.L.; Hau, S.K.; Jen, A.K.Y. Metal grid/conducting polymer hybrid transparent electrode for inverted polymer solar cells. Appl. Phys. Lett. 2010, 96, 203301. [Google Scholar] [CrossRef]
- Yang, B.Z.; Lin, Y.S.; Wu, J.M. Flexible contact-electrification field-effect transistor made from the P3HT:PCBM conductive polymer thin film. Appl. Mater. Today 2017, 9, 96–103. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Aswal, D.K. Conductive polymers for thermoelectric power generation. Prog. Mater. Sci. 2018, 93, 270–310. [Google Scholar] [CrossRef]
- Yang, R.B.; Reddy, P.M.; Chang, C.J.; Chen, P.A.; Chen, J.K.; Chang, C.C. Synthesis and characterization of Fe3O4/polypyrrole/carbon nanotube composites with tunable microwave absorption properties: Role of carbon nanotube and polypyrrole content. Chem. Eng. J. 2016, 285, 497–507. [Google Scholar] [CrossRef]
- Kang, H.; Park, H.; Park, Y.; Jung, M.; Kim, B.C.; Wallace, G.; Cho, G. Fully Roll-to-Roll Gravure Printable Wireless (13.56 MHz) Sensor-Signage Tags for Smart Packaging. Sci. Rep. 2014, 4, 5387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.J.; Tsai, M.H.; Hsu, Y.H.; Tuan, C.S. Morphology and Optoelectronic Property of ZnO rod array/Conjugated Polymer Hybrid Films. Thin Solid Films 2008, 516, 5523–5526. [Google Scholar] [CrossRef]
- Ates, M.; El-Kady, M.; Kaner, R.B. Three-dimensional design and fabrication of reduced graphene oxide/polyaniline composite hydrogel electrodes for high performance electrochemical supercapacitors. Nanotechnology 2018, 29, 175402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.J.; Leong, Y.Y.; Lai, C.F.; Chiou, W.Y.; Su, M.J.; Chang, S.J. Performance of white light emitting diodes prepared by casting wavelength-converting polymer on InGaN devices. J. Appl. Polym. Sci. 2017, 134, 45210. [Google Scholar] [CrossRef]
- Chang, C.J.; Lai, C.F.; Madhusudhana Reddy, P.; Chen, Y.L.; Chiou, W.Y.; Chang, S.J. Color optimization of conjugated-polymer/InGaN hybrid white light emitting diodes by incomplete energy transfer. J. Lumin. 2015, 160, 145–150. [Google Scholar] [CrossRef]
- Lai, C.F.; Chang, C.J.; Hsieh, C.L.; Chen, Y.L.; Tuan, C.S. Highly bright broadband red light produced by fluorescence polymer/InGaN hybrid light-emitting diodes. Opt. Lett. 2013, 38, 4082–4084. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, Y.; Ji, X.; Huang, S.; Xia, J.; Xie, M.; Yan, J.; Xu, H.; Li, H. Conjugated conducting polymers PANI decorated Bi12O17Cl2 photocatalyst with extended light response range and enhanced photoactivity. Appl. Surf. Sci. 2019, 464, 552–561. [Google Scholar] [CrossRef]
- Yu, W.J.; Cheng, Y.; Zou, T.; Liu, Y.; Wu, K.; Peng, N. Preparation of BiPO4-Polyaniline Hybrid and its Enhanced Photocatalytic Performance. Nano 2018, 13, 1850009. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Z.; Wang, W.; Ju, T.; She, H.; Wang, Q. Synthesis and characterization of polyaniline-modified BiOI: A visible-light-response photocatalyst. J. Mater. Sci. Mater. Electron. 2018, 29, 18343–18351. [Google Scholar] [CrossRef]
- Hao, X.; Gong, J.; Zhang, D.; Xiao, X.; Jiang, Y.; Zhang, F.; Tong, Z. Preparation of polyaniline modified BiOBr with enhanced photocatalytic activities. Funct. Mater. Lett. 2017, 10, 1750040. [Google Scholar] [CrossRef]
- Wang, Q.; Hui, J.; Li, J.; Cai, Y.; Yin, S.; Wang, F.; Su, B. Photodegradation of methyl orange with PANI-modified BiOCl photocatalyst under visible light irradiation. Appl. Surf. Sci. 2013, 283, 577–583. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Sun, S.; Ren, J.; Zhou, L.; Zhang, L. Efficient Visible Light-Induced Photocatalytic Degradation of Contaminant by Spindle-like PANI/BiVO4. J. Phys. Chem. C 2009, 113, 20228–20233. [Google Scholar] [CrossRef]
- Merkulov, D.V.Š.; Despotović, V.N.; Banić, N.D.; Armaković, S.J.; Finčur, N.L.; Lazerević, M.J.; Četojević-Simin, D.D.; Orčić, D.Z.; Radoičić, M.B.; Šaponjić, Z.V.; et al. Photocatalytic decomposition of selected biologically active compounds in environmental waters using TiO2/polyaniline nanocomposites: Kinetics, toxicity and intermediates assessment. Environ. Pollut. 2018, 239, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; An, W.; Li, Y.; Liang, Y.; Cui, W. Fabricating 3D porous PANI/TiO2–graphene hydrogel for the enhanced UV-light photocatalytic degradation of BPA. Appl. Surf. Sci. 2018, 27, 123–132. [Google Scholar] [CrossRef]
- Xu, S.; Han, Y.; Xu, Y.; Meng, H.; Xu, J.; Wu, J.; Xu, Y.; Zhang, X. Fabrication of polyaniline sensitized grey-TiO2 nanocomposites and enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 184, 248–256. [Google Scholar] [CrossRef]
- Gilja, V.; Novaković, K.; Travas-Sejdic, J.; Hrnjak-Murgić, Z.; Roković, M.K.; Zic, M. Stability and Synergistic Effect of Polyaniline/TiO2 Photocatalysts in Degradation of Azo Dye in Wastewater. Nanomaterials 2017, 7, 412. [Google Scholar] [CrossRef]
- Razak, S.; Nawi, M.A.; Haitham, K. Fabrication, characterization and application of a reusable immobilized TiO2–PANI photocatalyst plate for the removal of reactive red 4 dye. Appl. Surf. Sci. 2014, 319, 90–98. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Zeng, G.; Dong, H.; Yan, M.; Wang, J.; Hu, W.; Wang, J.; Zhou, Y.; Tang, J. Enhanced visible light photocatalytic performance of polyaniline modified mesoporous single crystal TiO2 microsphere. Appl. Surf. Sci. 2016, 387, 882–893. [Google Scholar] [CrossRef]
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Cho, M.H. Electrically conductive polyaniline sensitized defective-TiO2 for improved visible light photocatalytic and photoelectrochemical performance: A synergistic effect. New J. Chem. 2015, 39, 8381–8388. [Google Scholar] [CrossRef]
- Jeong, W.-H.; Amna, T.; Ha, Y.M.; Hassan, M.S.; Kim, H.C.; Khil, M.-S. Novel PANI nanotube@TiO2 composite as efficient chemical and biological disinfectant. Chem. Eng. J. 2014, 246, 204–210. [Google Scholar] [CrossRef]
- Guo, N.; Liang, Y.; Lan, S.; Liu, L.; Zhang, J.; Ji, G.; Gan, S. Microscale Hierarchical Three-Dimensional Flowerlike TiO2/PANI Composite: Synthesis, Characterization, and Its Remarkable Photocatalytic Activity on Organic Dyes under UV-Light and Sunlight Irradiation. J. Phys. Chem. C 2014, 118, 18343–18355. [Google Scholar] [CrossRef]
- Wang, F.; Min, S.; Han, Y.; Feng, L. Visible-light-induced photocatalytic degradation of methylene blue with polyaniline-sensitized TiO2 composite photocatalysts. Superlattices Microstruct. 2010, 48, 170–180. [Google Scholar] [CrossRef]
- Wang, F.; Min, S.X. TiO2/polyaniline composites: An efficient photocatalyst for the degradation of methylene blue under natural light. Chin. Chem. Lett. 2007, 18, 1273–1277. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Jiao, X.; Chen, D. Synthesis and photocatalytic properties of flower-like zirconia nanostructures. CrystEngComm 2012, 14, 1122–1127. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R.; Gomathisankar, P. Phenol-photodegradation on ZrO2. Enhancement by semiconductors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 92, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Carević, M.V.; Abazović, N.D.; Novaković, T.B.; Pavlović, V.B.; Čomor, M.I. Zirconium dioxide nanopowders with incorporated Si4+ ions as efficient photocatalyst for degradation of trichlorophenol using simulated solar light. Appl. Catal. B Environ. 2016, 195, 112–120. [Google Scholar] [CrossRef]
- Carević, M.V.; Abazović, N.D.; Mitrić, G.; Ćirić-Marjanović, M.D.; Mojović, S.P.; Ahrenkiel, S.P.; Čomor, M.I. Properties of Zirconia/Polyaniline hybrid nanocomposites and their application as photocatalysts for degradation of model pollutants. Mater. Chem. Phys. 2018, 205, 130–137. [Google Scholar] [CrossRef]
- Chang, C.J.; Weng, H.T.; Chang, C.C. CuS/ZnS-g-C3N4 heterostructured photocatalysts for efficient photocatalytic hydrogen production. Int. J. Hydrog. Energy 2017, 42, 23568–23577. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, W.; Zong, R.; Yao, W.; Li, Z.; Zhu, Y. Polyaniline/Carbon Nitride Nanosheets Composite Hydrogel: A Separation-Free and High-Efficient Photocatalyst with 3D Hierarchical Structure. Small 2016, 12, 4370–4378. [Google Scholar] [CrossRef]
- Zeng, S.; Yang, J.; Qiu, X.; Liang, Z.; Zhang, Y. Magnetically recyclable MnFe2O4/polyaniline composite with enhanced visible light photocatalytic activity for rhodamine B degradation. J. Ceram. Soc. Jpn. 2016, 124, 1152–1156. [Google Scholar] [CrossRef]
- Kim, K.N.; Jung, H.R.; Lee, W.J. Hollow cobalt ferrite–polyaniline nanofibers as magnetically separable visible-light photocatalyst for photodegradation of methyl orange. J. Photochem. Photobiol. Chem. 2016, 321, 257–265. [Google Scholar] [CrossRef]
- Wu, H.; Lin, S.; Chen, C.; Liang, W.; Liu, X.; Yang, H. A new ZnO/rGO/polyaniline ternary nanocomposite as photocatalyst with improved photocatalytic activity. Mater. Res. Bull. 2016, 83, 434–441. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z. Role of Polyaniline on the Photocatalytic Degradation and Stability Performance of the Polyaniline/Silver/Silver Phosphate Composite under Visible Light. ACS Appl. Mater. Interfaces 2014, 6, 17589–17598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, L.; Zeng, M.; Li, J.; Xu, J.; Wang, X. Hierarchical nanocomposites of polyaniline nanorods arrays on graphitic carbon nitride sheets with synergistic effect for photocatalysis. Catal. Today 2014, 224, 114–121. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Lu, M.; Fan, Z.; Weng, S.; Hu, J.; Liu, P. Synergistic Effect in Polyaniline-Hybrid Defective ZnO with Enhanced Photocatalytic Activity and Stability. J. Phys. Chem. C 2014, 118, 9570–9577. [Google Scholar] [CrossRef]
- Eskizeybek, V.; Sarı, F.; Gülce, H.; Gülce, A.; Avcı, A. Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Appl. Catal. B Environ. 2012, 119–120, 197–206. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y. Significant Visible Photoactivity and Antiphotocorrosion Performance of CdS Photocatalysts after Monolayer Polyaniline Hybridization. J. Phys. Chem. C 2010, 114, 5822–5826. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Z.Y.; Zhao, H.; Yu, W.; Wu, S.; Liu, J.; Chen, L.; Li, Y. Probing conducting polymers@cadmium sulfide core-shell nanorods for highly improved photocatalytic hydrogen production. J. Colloid Interface Sci. 2018, 521, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Kato, H.; Kudo, A. New tantalate photocatalysts for water decomposition into H2 and O2. Chem. Phys. Lett. 1998, 295, 487–492. [Google Scholar] [CrossRef]
- Zielińska, B.; Schmidt, B.; Mijowska, E.; Kaleńczuk, R. PANI/NaTaO3 composite photocatalyst for enhanced hydrogen generation under UV light irradiation. Pol. J. Chem. Technol. 2017, 19, 115–119. [Google Scholar] [CrossRef]
- Chang, C.J.; Chu, K.W. ZnS/polyaniline composites with improved dispersing stability and high photocatalytic hydrogen production activity. Int. J. Hydrog. Energy 2016, 41, 21764–21773. [Google Scholar] [CrossRef]
- Nsib, M.F.; Saafi, S.; Rayes, A.; Houas, A. Synthesis and characterization of the hybrid Ni-TiO2/PANI for an efficient hydrogen photoproduction under visible light. Sci. Technol. Sci. Exactes 2014, 39, 107–117. [Google Scholar]
- Wang, X.; Feng, S.; Zhao, W.; Zhao, D.; Chen, S. Ag/polyaniline heterostructured nanosheets loaded with g-C3N4 nanoparticles for highly efficient photocatalytic hydrogen generation under visible light. New J. Chem. 2017, 41, 9354–9360. [Google Scholar] [CrossRef]
- Sasikala, R.; Gaikwad, A.P.; Jayakumar, O.D.; Girija, K.G.; Rao, R.; Tyagi, A.K.; Bharadwaj, S.R. Nanohybrid MoS2-PANI-CdS photocatalyst for hydrogen evolution from water. Colloids Surf. Physicochem. Eng. Asp. 2015, 481, 485–492. [Google Scholar] [CrossRef]
- Yuan, X.; Floresyona, D.; Aubert, P.H.; Bui, T.T.; Remita, S.; Ghosh, S.; Brisset, F.; Goubard, F.; Remita, H. Photocatalytic degradation of organic pollutant with polypyrrole nanostructures under UV and visible light. Appl. Catal. B Environ. 2019, 242, 284–292. [Google Scholar] [CrossRef]
- Shan, Y.; Zhao, J.; Li, W.; Huang, Q.; Xiao, C. Dual effect of polypyrrole doping on cadmium sulfide for enhanced photocatalytic activity and robust photostability. J. Mater. Sci. 2018, 53, 2065–2076. [Google Scholar] [CrossRef]
- Baig, U.; Gondal, M.A.; Ilyas, A.M.; Sanagi, M.M. Band gap engineered polymeric-inorganic nanocomposite catalysts: Synthesis, isothermal stability, photocatalytic activity and photovoltaic performance. J. Mater. Sci. Technol. 2017, 33, 547–557. [Google Scholar] [CrossRef]
- Gao, F.; Hou, X.; Wang, A.; Chu, G.; Wu, W.; Chen, J.; Zou, H. Preparation of polypyrrole/TiO2 nanocomposites with enhanced photocatalytic performance. Particuology 2016, 26, 73–78. [Google Scholar] [CrossRef]
- Liu, X.; Cai, L. Novel indirect Z-scheme photocatalyst of Ag nanoparticles and polymer polypyrrole co-modified BiOBr for photocatalytic decomposition of organic pollutants. Appl. Surf. Sci. 2018, 445, 242–254. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, L.; Chen, Z.; Jing, H.; Chen, Y.; Li, Q. Synthesis of magnetic photocatalyst and sensitization properties of polypyrrole. Sci. Eng. Compos. Mater. 2014, 23, 269–275. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Y.; Zeng, C.; Zhang, Y.; Huang, H. Polypyrrole decorated BiOI nanosheets: Efficient photocatalytic activity for treating diverse contaminants and the critical role of bifunctional polypyrrole. J. Colloid Interface Sci. 2017, 50, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, W.; Gao, E. Polypyrrole/Bi2WO6 composite with high charge separation efficiency and enhanced photocatalytic activity. J. Mater. Sci. 2014, 49, 7325–7332. [Google Scholar] [CrossRef]
- Gao, B.; Chen, W.; Dong, S.; Liu, J.; Liu, T.; Wang, L.; Sillanpää, M. Polypyrrole/ZnIn2S4 composite photocatalyst for enhanced mineralization of chloramphenicol under visible light. J. Photochem. Photobiol. Chem. 2017, 349, 115–123. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhu, P.P.; Li, X.; Zhou, K.F.; Yang, J.B.; Ma, X.L.; Sha, J.Q. Construction, PPy loading and photocatalytic performance of a polyoxometalate-templated metallacycle compound. J. Coord. Chem. 2017, 70, 3353–3362. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Zhang, Q.; Zhu, X.; Dai, Y. Precisely locate Pd-Polypyrrole on TiO2 for enhanced hydrogen production. Int. J. Hydrog. Energy 2017, 42, 25195–25202. [Google Scholar] [CrossRef]
- Chandra, M.R.; Rao, T.S.; Kim, H.-S.; Pammi, S.V.; Prabhakarrao, N.N.; Manga Raju, I. Hybrid copper doped titania/polythiophene nanorods as efficient visible light-driven photocatalyst for degradation of organic pollutants. J. Asian Ceram. Soc. 2017, 5, 436–443. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Q.; Yu, X.; Lu, Q.; Hong, X. Highly Efficient Metal-Free Visible Light Driven Photocatalyst: Graphene Oxide/Polythiophene Composite. Chem. Sel. 2017, 2, 5578–5586. [Google Scholar] [CrossRef]
- Dai, W.; Xu, H.; Yu, J.; Hu, X.; Luo, X.; Tu, X.; Yang, L. Photocatalytic reduction of CO2 into methanol and ethanol over conducting polymers modified Bi2WO6 microspheres under visible light. Appl. Surf. Sci. 2015, 356, 173–180. [Google Scholar] [CrossRef]









| Photocatalyst | Structure | Synthesis Method | Light Source | Model Pollutants | Degradation Efficiency/Time | Ref. (Year) |
|---|---|---|---|---|---|---|
| PANI/Bi12O17Cl2 | Irregular nanosheets | In-situ synthesis | 300-W Xe lamp (vis) (λ > 420nm) | Ciprofloxacin, Rhodamine B | CIP: 90.4%/120 min RhB: 99%/100 min | [17] (2019) |
| BiPO4-PANI | heterostructure | Hydrothermal and hybridization | 300-W Xe lamp (vis) (λ > 420nm) | Methylene blue | 87.3%/120 min | [18] (2018) |
| PANI/BiOI | Nanoplates/nanoparticles | Co-precipitation | 300-W Xe lamp (vis) (λ = 420nm) | Rhodamine B | 91%/120 min | [19] (2018) |
| PANI/BiOBr-0.2 | Nanofibers | Hydrothermal | 350-W Xe lamp (simulated sunlight) | Rhodamine B | 99.9%/75 min | [20] (2017) |
| PANI/BiOCl | Nanosheets | Chemisorption | 500-W high pressure Xe lamp (vis) (λ > 420nm) | Methyl orange | 67%/20 min | [21] (2013) |
| PANI/BiVO4 | Spindle like structure | Sonochemical | 500-W Xe lamp (vis) (λ > 420nm) | Rhodamine B, phenol | RhB: 100%/60 min Phenol: 24%/120 min | [22] (2009) |
| Photocatalyst | Structure | Synthesis Method | Light Source | Model Pollutants | Degradation Efficiency/Time | Ref. (Year) |
|---|---|---|---|---|---|---|
| TiO2/PANI | Nanocomposites | Polymerization | Hg-lamp (UV) | Pharmaceutical: Propranolol, amitriptyline; Pesticides: sulcotrione, clomazone | Propranolol: 23%/60 min Amitriptyline: 45%/60 min Sulcotrione: 94%/60 min Clomazone: 32 %/60 min | [23] (2018) |
| PANI/TiO2-rGH | Composite hydrogel | Chemical reduction | 500-W Hg-lamp (UV) | Bisphenol A | 80.5%/70 min | [24] (2018) |
| PANI/grey-TiO2 | Nanocomposite | In-situ polymerization | 300-W Xe lamp (vis) (λ = 420 nm) | Rhodamine B | ~100%/180 min | [25] (2017) |
| PANI/TiO2 | Nanocomposites | In-situ polymerization | Pen-Ray UYP lamp (UVA) (315 nm < λ < 400 nm) | Reactive Red 45 | 94%/60 min | [26] (2017) |
| TiO2/PANI | core-shell composites | Dip-coating | 45-W indoor fluorescent lamp (UV) | Reactive Red 45 | 85%/60 min | [27] (2014) |
| PANI/MS-TiO2 | Mesoporous crystal microsphere | Solution evaporation and chemisorption | 300-W Xe lamp (vis) (λ > 420nm) | Rhodamine B and Methylene blue | RhB: 99.8%/120 min MB: 99.5%/150 min | [28] (2016) |
| s-PANI@m-TiO2 | Nanocomposites | In-situ polymerization | 400-W lamp (vis) | Methylene blue, Brilliant blue | MB: ~100%/420 min BB: ~100%/300 min | [29] (2015) |
| PANI-TiO2 | Nanotube-nanoparticles | Polymerization | (vis) (λ > 400nm) | Methylene blue | 85%/300 min | [30] (2014) |
| TiO2/PANI | Microscale hierarchical 3D flowerlike | In-situ polymerization | Natural sunlight irradiation | Congo red and Methyl orange | CR: 96%/120 min MO: 90%/120 min | [31] (2014) |
| PANI/TiO2 | Nanocomposites | Simple solution method | 110-W high pressure Na lamp (vis) (λ > 400nm) | Methylene blue | 81.74%/120 min | [32] (2010) |
| TiO2/PANI | Nanocrystalline composites | In-situ polymerization | Natural light | Methylene blue | ~90%/90 min | [33] (2007) |
| Photocatalyst | Structure | Synthesis Method | Light Source | Model Pollutants | Degradation Efficiency/Time | Ref. (Year) |
|---|---|---|---|---|---|---|
| ZrO2/PANI | Hybrid nanocomposite | polymerization | 300-W white light lamp (λ = 280 to 315 nm) | Trichlorophenol | 75%/210 min | [39] (2018) |
| PANI/CNNS | 3D hierarchical composite hydrogels | In-situ polymerization | 500-W Xe lamp (vis) (λ > 420nm) | Methylene blue | 89.1%/240 min | [40] (2016) |
| MnFe2O4/PANI | Nanocomposite | In-situ polymerization | 175-W halide lamp (λ < 400 nm) | Rhodamine B | ~100%/60 min | [41] (2016) |
| CoFe2O4/PANI | Hollow nanofibers/nanograins | In-situ polymerization | 10-W LED lamp (vis) | Methyl orange | 85%/120 min | [42] (2016) |
| ZnO/rGO/PANI | Nanocomposite | In-situ polymerization | 500-W high pressure Hg lamp (UV) | Methyl orange | ~100%/60 min | [43] (2016) |
| PANI/Ag/Ag3PO4 | Composites | In-situ deposition | 300-W Xe lamp (vis) (λ < 420 nm) | Rhodamine B | >95%/5 min | [44] (2014) |
| g-C3N4/PANI | Nanosheets/nanorods | polymerization | 500-W Xe lamp (vis) (400 nm < λ < 700 nm) | Methylene blue and methyl orange | MB: 78.6%/30 min MO: 99.8%/30 min | [45] (2014) |
| Hybridized defective ZnO/PANI | Hybridized nanocrystal | Chemisorption and cold plasma treatment (CPT) | 4-W UV lamp (UV) (λ = 365 nm) | Methyl orange and 4-chlorophenol | MO: ~93.6%/120 min 4-CP: ~87.8%/120 min | [46] (2014) |
| PANI/ZnO | Nanocomposite | Chemical oxidative polymerization | Natural sunlight (10:00 a.m. to 3.00 p.m.) | Methylene blue and malachite green | MB: 97%/300 min MG: 99%/300 min | [47] (2012) |
| PANI/CdS | Monolayer-hybrid | Chemisorption | 500-W Xe lamp (vis) (λ > 450 nm) | Methylene blue | 92%/300 min | [48] (2010) |
| Photocatalyst | Structure | Synthetic Method | Light Source | Sacrificial Agent | Activity (μmol h−1g−1) | Ref. (Year) |
|---|---|---|---|---|---|---|
| PANI@CdS | Core-shell nanorods | In-situ polymerization | PLS-SXE-300C lamp (vis) (λ ≥ 420 nm) | 0.25 M Na2SO3 + 0.35 M Na2S | ~9700 | [49] (2018) |
| PANI/g-TiO2 | Nanocomposites | In-situ polymerization | 300-W Xe (vis) | Methanol | 1790 | [25] (2017) |
| PANI/NaTaO3 | Nanocomposites | Polymerization | 150-W Hg lamp (UV) | Formic acid | 163 μmole h−1 | [52] (2017) |
| PANI-ZnS | Nanocomposites | Solvothermal | Hg lamp (UV) | 0.1 M Na2S2 + 0.04 M Na2SO3 + 3 M NaCl | 6750 μmol h−1 g−1 | [53] (2016) |
| Ni-ZnO/PANI | Nanorods-like | Direct impregnation | 250-W Halogen visible lamp (vis) | 0.2 M Na2S2O3 + Na2CO3 | ~558 558 μmole h−1 | [54] (2014) |
| g-C3N4-Ag/PANI | Heterostructured nanosheets | Sonication + hydrothermal | 300-W Xe lamp (vis) (λ < 420 nm) | Methanol | 210.73 μmol h−1 mg−1 | [55] (2017) |
| MoS2-PANI-CdS | Hybrid structure | Sonication | Day-light fluorescent lamps | 0.8 M Na2SO3 + 0.6 M Na2S | ~78 μmol | [56] (2015) |
| Photocatalyst | Synthesis Method | Light Source | Model Pollutants | Degradation Efficiency/Time | Ref. (Year) |
|---|---|---|---|---|---|
| PPy | Radiolysis/polymerization | 300-W Xe lamp (vis) (λ < 420 nm) | Phenol, methyl orange | Phenol: 20%/300 min MO: 80%/300 min | [57] (2019) |
| PPy-CdS | In-situ polymerization | Xe lamp | Rhodamine B, Methylene blue | RhB: 99.34%/150 min MB: 99.93%/150 min | [58] (2018) |
| PPy-TiO2 | polymerization | 500-W Xe lamp (vis) | Methyl orange | 100%/60 min | [59] (2017) |
| PPy/TiO2 | polymerization | 500-W tungsten-halogen lamp | Rhodamine B | 97%/480 min | [60] (2016) |
| BiOBr-Ag-PPy | polymerization | 150-W halogen lamp (vis) (λ < 420 nm) | Malachite green | 97%/120 min | [61] (2018) |
| PPy-TiO2/M-Fe2O3 | In-situ polymerization | Solar radiation | Methyl orange | 59.3%/90 min | [62] (2014) |
| PPy-BiOI | In-situ precipitation | (vis) (λ > 420 nm) | Rhodamine B | 83%/300 min | [63] (2017) |
| PPy/Bi2WO6 | Photocatalytic oxidative polymerization | 500-W Xe lamp (vis) | Phenol | ~100%/120 min | [64] (2014) |
| PPy-ZnIn2S4 | Hydrothermal | 100-W Iodine-gallium lamp | Chloramphenicol | 100%/60 min | [65] (2017) |
| PPy-AgPMo12 | In-situ polymerization | 300-W Xe lamp (vis) | Rhodamine B | 73.09%/360 min | [66] (2017) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.L.; Chang, C.-J. Recent Developments about Conductive Polymer Based Composite Photocatalysts. Polymers 2019, 11, 206. https://doi.org/10.3390/polym11020206
Lee SL, Chang C-J. Recent Developments about Conductive Polymer Based Composite Photocatalysts. Polymers. 2019; 11(2):206. https://doi.org/10.3390/polym11020206
Chicago/Turabian StyleLee, Sher Ling, and Chi-Jung Chang. 2019. "Recent Developments about Conductive Polymer Based Composite Photocatalysts" Polymers 11, no. 2: 206. https://doi.org/10.3390/polym11020206
APA StyleLee, S. L., & Chang, C. -J. (2019). Recent Developments about Conductive Polymer Based Composite Photocatalysts. Polymers, 11(2), 206. https://doi.org/10.3390/polym11020206