Polypyrrole Nanowires with Ordered Large Mesopores: Synthesis, Characterization and Applications in Supercapacitor and Lithium/Sulfur Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.1.1. Chemical Synthesis
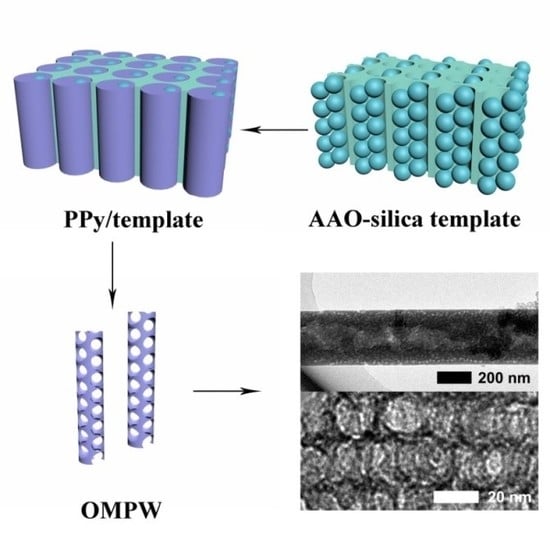
2.1.2. Preparation of AAO-Silica Template
2.1.3. Preparation of OMPW
2.2. Material Characterization
2.3. Electrode Preparation and Electrochemical Tests
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sharma, M.; Waterhouse, G.I.N.; Loader, S.W.C.; Garg, S.; Svirskis, D. High surface area polypyrrole scaffolds for tunable drug delivery. Int. J. Pharm. 2013, 443, 163–168. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Polyaniline/Pt hybrid nanofibers: High-efficiency nanoelectrocatalysts for electrochemical devices. Small 2009, 5, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Lei, W.; He, P.; Wang, Y.; Zhang, S.; Dong, F.; Liu, H. Soft template interfacial growth of novel ultralong polypyrrole nanowires for electrochemical energy storage. Electrochim. Acta 2014, 132, 112–117. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, J.; Zhao, X.; Tu, J.; Su, Q.; Du, G. Sulfur@hollow polypyrrole sphere nanocomposites for rechargeable Li-S batteries. RSC Adv. 2013, 3, 24914–24917. [Google Scholar] [CrossRef]
- Yin, F.; Wang, D.; Zhang, Z.; Zhang, C.; Zhang, Y. Synthesis of mesoporous hollow polypyrrole spheres and the utilization as supports of high loading of Pt nanoparticles. Mater. Lett. 2017, 207, 225–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, J.; Wang, D.; Zhang, C.; Yin, F.; Mukanova, A.; Bakenov, Z. Sulfur-infiltrated three-dimensionally ordered mesoporous polypyrrole cathode for high-performance lithium-sulfur battery. ChemElectroChem 2018, 5, 1591–1598. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Ren, J.; Zhang, W.; Zhang, C.; Zhang, Y. Three-dimensionally ordered hierarchically porous polypyrrole loading sulfur as high-performance cathode for lithium/sulfur batteries. Polymer 2018, 137, 261–268. [Google Scholar] [CrossRef]
- Huang, Z.H.; Song, Y.; Xu, X.; Liu, X.X. Ordered polypyrrole nanowire arrays grown on carbon cloth substrate for high performance pseudocapacitor electrode. ACS Appl. Mater. Interface 2015, 7, 25506–25513. [Google Scholar] [CrossRef]
- Yin, F.; Liu, X.; Zhang, Y.; Zhao, Y.; Menbayeva, A.; Bakenov, Z.; Wang, X. Well-dispersed sulfur anchored on interconnected polypyrrole nanofiber network as high performance cathode for lithium-sulfur batteries. Solid State Sci. 2017, 66, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Nie, P.; Le, Z.; Chen, G.; Liu, D.; Liu, X.; Wu, H.; Xu, P. Graphene Caging Silicon Particles for High-Performance Lithium-Ion Batteries. Small 2018, 14, 1800635–1800642. [Google Scholar] [CrossRef]
- Peng, Y.; Le, Z.; Wen, M.; Zhang, D.; Chen, Z.; Wu, H.; Li, H.; Lu, Y. Mesoporous single-crystal-like TiO2 mesocages threaded with carbon nanotubes for high-performance electrochemical energy storage. Nano Energy 2017, 35, 44–51. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, L.; Yan, Y.; Chen, J. Controlled synthesis of Pt nanowires with ordered large mesopores for methanol oxidation reaction. Sci. Rep. 2016, 6, 31440–31447. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cheng, G.; Katsov, K.; Sides, S.W.; Wang, J.; Tang, J.; Fredrickson, G.H.; Moskovits, M.; Stucky, G.D. Composite mesostructures by nano-confinement. Nat. Mater. 2004, 3, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, G.; Zhang, C.; Yin, F. Enhanced electrocatalytic activity and durability of Pt nanoparticles supported on ordered bimodal mesoporous carbon nanowires. Mater. Lett. 2018, 228, 92–95. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Wen, Z.; Huang, L.; Wang, X.; Zhang, H. A nano-structured and highly ordered polypyrrole-sulfur cathode for lithium–sulfur batteries. J. Power Sources 2011, 196, 6951–6955. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of conducting polymers-persistent models and new concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, D.; Pan, J.; Cao, Y.; Yang, H.; Ai, X. A Li+-conductive microporous carbon-sulfur composite for Li-S batteries. Electrochim. Acta 2013, 87, 497–502. [Google Scholar] [CrossRef]
- Ghosh, S.; Bowmaker, G.A.; Cooney, R.P.; Seakins, J.M. Infrared and Raman spectroscopic studies of the electrochemical oxidative degradation of polypyrrole. Synthetic Met. 1998, 20, 63–67. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, L.C.; Wang, D.W.; Li, L.; Pei, S.; Gentle, I.R.; Li, F.; Cheng, H.M. Fibrous hybrid of graphene and sulfur nanocrystals for high-performance lithium-sulfur batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Rui, K.; Wu, X.; Wu, M.; Zhang, J. Enhanced performance of lithium sulfur battery with polypyrrole warped mesoporous carbon/sulfur composite. J. Power Sources 2014, 254, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Singu, B.S.; Yoon, K.R. Highly exfoliated GO-PPy-Ag ternary nanocomposite for electrochemical supercapacitor. Electrochim. Acta 2018, 268, 304–315. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Le, Z.; Liu, F.; Nie, P.; Li, X.; Liu, X.; Bian, Z.; Chen, G.; Wu, H.; Lu, Y. Pseudocapacitive Sodium Storage in Mesoporous Single-Crystal-like TiO2−Graphene Nanocomposite Enables High Performance Sodium-Ion Capacitors. ACS Nano 2017, 11, 2952–2960. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, S.; Guan, C. Polypyrrole Nanowires Coated with a Hollow Shell for Enhanced Electrochemical Performance. Mater. Res. Bull. 2018, 100, 116–119. [Google Scholar] [CrossRef]
- Mao, L.; Li, M.; Xue, J.; Wang, J. Bendable graphene/conducting polymer hybrid films for freestanding electrodes with high volumetric capacitances. RSC Adv. 2016, 6, 2951–2957. [Google Scholar] [CrossRef]
- Santino, L.; Hwang, E.; Diao, Y.; Lu, Y.; Wang, H.; Jiang, Q.; Singamaneni, S.; D’Arcy, J. Condensing Vapor Phase Polymerization (CVPP) of Electrochemically Capacitive and Stable Polypyrrole Microtubes. ACS Appl. Mater. Interface 2017, 9, 41496–41504. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Wei, Z. Conducting polymer nanowire arrays with enhanced electrochemical performance. J. Mater. Chem. 2010, 20, 1117–1121. [Google Scholar] [CrossRef]
- An, H.; Wang, Y.; Wang, X.; Zheng, L.; Wang, X.; Yi, L.; Bai, L.; Zhang, X. Polypyrrole/carbon aerogel composite materials for supercapacitor. J. Power Sources 2010, 195, 6964–6969. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, B.; Chen, J.; Li, F.; Liu, K.; Wang, Y.; Li, M.; Lu, Z.; Wang, W.; Wang, D. Facile synthesis of three-dimensional (3D) interconnecting polypyrrole (PPy) nanowires/nanofibrous textile composite electrode for high performance supercapacitors. Compos. Part A Appl. S 2017, 101, 30–40. [Google Scholar] [CrossRef]
- Ghenaatian, H.R.; Mousavi, M.F.; Rahmanifar, M.S. High performance hybrid supercapacitor based on two nanostructured conducting polymers: Self-doped polyaniline and polypyrrole nanofibers. Electrochim. Acta 2012, 78, 212–222. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhu, J.; Sun, X.; Cheng, Z.; Liu, Y.; Wang, Y. High density of free-standing holey graphene/PPy films for superior volumetric capacitance of supercapacitors. ACS Appl. Mater. Interface 2017, 9, 21763–21772. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Drzal, L.T. Multilayered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem. Mater. 2010, 22, 5667–5671. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Wang, J.; Wang, J.; Bai, Y.; Du, X. Morphology controllable nano-sheet polypyrrole-graphene composites for high-rate supercapacitor. Phys. Chem. Chem. Phys. 2015, 17, 19885–19894. [Google Scholar] [PubMed]
- Fan, L.Q.; Liu, G.J.; Wu, J.H.; Liu, L.; Lin, J.M.; Wei, Y.L. Asymmetric supercapacitor based on graphene oxide/polypyrrole composite and activated carbon electrodes. Electrochim. Acta 2014, 137, 26–33. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Chen, J.; Li, X.; Walsh, F.; Ouyang, J.; Jia, D.; Zhou, Y. Three-dimensional graphene oxide/polypyrrole composite electrodes fabricated by one-step electrodeposition for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 14445–14457. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Cao, J.; Liu, Y.; Zhou, Y.; Quang, J.; Jia, D. Facile Co-Electrodeposition Method for High-Performance Supercapacitor Based on Reduced Graphene Oxide/Polypyrrole Composite Film. ACS Appl. Mater. Interface 2017, 9, 19831–19842. [Google Scholar] [CrossRef]
- Qian, W.; Gao, Q.; Zhang, H.; Tian, W.; Li, Z.; Tan, Y. Crosslinked polypyrrole grafted reduced graphene oxide-sulfur nanocomposite cathode for high performance Li-S battery. Electrochim. Acta 2017, 235, 32–41. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, M.; Kaiser, M.R.; Gao, X.; Konstantinov, K.; Tandiono, R.; Wang, Z.; Liu, H.K.; Dou, S.X.; Wang, J. Split-half-tubular polypyrrole@sulfur@polypyrrole composite with a novel three-layer-3D structure as cathode for lithium/sulfur batteries. Nano Energy 2015, 11, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhao, H.; Cheng, H.; Hu, C.; Fang, W.; Fang, J.; Xu, J.; Chen, Z. Facile large-scale synthesis of core–shell structured sulfur@polypyrrole composite and its application in lithium-sulfur batteries with high energy density. Appl. Energy 2016, 175, 522–528. [Google Scholar] [CrossRef]
- Fu, Y.; Manthiram, A. Orthorhombic bipyramidal sulfur coated with polypyrrole nanolayers as a cathode material for lithium-sulfur batteries. J. Phys. Chem. C 2012, 116, 8910–8915. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Wu, M.; Wu, X.; Zhang, J. Enhanced cycle performance of Li-S battery with a polypyrrole functional interlayer. J. Power Sources 2014, 267, 542–546. [Google Scholar] [CrossRef]







| Cycle number | Ro [Ω] | Rs [Ω] | Rct [Ω] |
|---|---|---|---|
| Fresh cell | 3.8 | - | 197.5 |
| 1st | 5.2 | 33.5 | 100.3 |
| 5th | 2.9 | 14 | 31.9 |
| 50th | 3.2 | 11.4 | 15.7 |
| 100th | 4 | 15.5 | 13.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Ren, J.; Wu, G.; Zhang, C.; Zhang, Y. Polypyrrole Nanowires with Ordered Large Mesopores: Synthesis, Characterization and Applications in Supercapacitor and Lithium/Sulfur Batteries. Polymers 2019, 11, 277. https://doi.org/10.3390/polym11020277
Yin F, Ren J, Wu G, Zhang C, Zhang Y. Polypyrrole Nanowires with Ordered Large Mesopores: Synthesis, Characterization and Applications in Supercapacitor and Lithium/Sulfur Batteries. Polymers. 2019; 11(2):277. https://doi.org/10.3390/polym11020277
Chicago/Turabian StyleYin, Fuxing, Jun Ren, Guoyan Wu, Chengwei Zhang, and Yongguang Zhang. 2019. "Polypyrrole Nanowires with Ordered Large Mesopores: Synthesis, Characterization and Applications in Supercapacitor and Lithium/Sulfur Batteries" Polymers 11, no. 2: 277. https://doi.org/10.3390/polym11020277
APA StyleYin, F., Ren, J., Wu, G., Zhang, C., & Zhang, Y. (2019). Polypyrrole Nanowires with Ordered Large Mesopores: Synthesis, Characterization and Applications in Supercapacitor and Lithium/Sulfur Batteries. Polymers, 11(2), 277. https://doi.org/10.3390/polym11020277