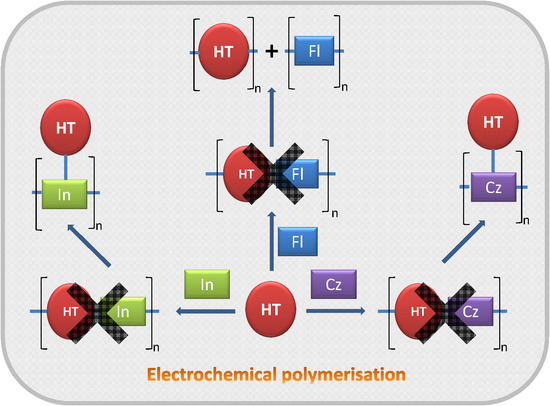
The Different Outcomes of Electrochemical Copolymerisation: 3-Hexylthiophene with Indole, Carbazole or Fluorene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Polymerisation and Spectroelectroconductometric Meausrements
- Standard cyclic voltammetry measurements—we used glass plates, on whose surface two Pt working electrodes were deposited in an interdigitated array configuration. The interdigitated section consisted of 500 whisker pairs, with a Pt path width of 5 μm and path spacing of 5 μm (Dropsens, Oviedo, Spain);
- IR spectroscopic investigations—Pt flag working electrodes were used, as they were able to deform without breaking when pressed to the surface of the attenuated total reflection (ATR) crystal of the IR spectrometer;
- Ultraviolet-visible-near infrared (UV-Vis-NIR) spectroscopic measurements—we used thin glass slides (90 mm × 7 mm × 0.3 mm), on whose surface two Pt working electrodes were deposited in an interdigitated array configuration. The design of the interdigitated section was identical to the one detailed above; the electrodes were custom-produced by Micrux (Oviedo, Spain).
3. Results and Discussion
3.1. Electrochemical Polymerisation and Cyclic Voltammetry of Layers Obtained
3.2. Material Identification
3.2.1. Polyindole
3.2.2. Polycarbazole
3.2.3. Polyfluorene
3.2.4. Discussion of Infrared (IR) and Raman Spectroscopic Results
3.3. Ultraviolet-Visible-Near Infrared (UV-Vis-NIR) Spectra of the Produced Films
3.4. Copolymerisation in Boron Trifluoride Diethyl Etherate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

| Copolymers/Polymers | Functional Group/Assignments |
|---|---|
| In; In EP; In-HT EP | νIn C–H 740 cm−1; νHT C–H 992 cm−1; νBF4- 998 cm−1; νIn C–N 1197 cm−1; νIn C–N 1248 cm−1; νIn C–N–C–C 1310 cm−1; νIn N–H 1460 cm−1; νHT C=C 1493 cm−1; νAr C–C 1570 cm−1; νIn Ar C–C 1596 cm−1; νHT C–H 2870 cm−1; νAr C–H 3058 cm−1; νIn N–H 3410 cm−1 |
| Cz; Cz EP; Cz-HT EP | νcz C–H 740 cm−1; νHT C–H 810 cm−1; νCz C3–C6 886 cm−1; νBF4- 998 cm−1; νCz C–N 1205 cm−1; νCz C–N–C 1238 cm−1; νCz N–H 1334 cm−1; νCz C–C 1397 cm−1; νHT C=C 1450 cm−1; ν HT C=C 1500 cm−1; νIn Ar C–C 1548 cm−1; νIn Ar C–C 1604 cm−1; νHT C–H 2870 cm−1; νAr C–H 3058 cm−1; νIn N–H 3426 cm−1 |
| Fl; Fl EP; Fl-HT EP | νFl C–H 720 cm−1; νHT C–H 820 cm−1; νFl C–H 890 cm−1; νBF4- 998 cm−1; νFl C–C 1185 cm−1; νFl C–C 1295 cm−1; νHT C=C 1450 cm−1; νFlz C–H 1456 cm−1; νFl Ar C–C 1600 cm−1; νFl Ar C–C 1660 cm−1; νFl Ar C–C 1713 cm−1; νHT C–H 2960 cm−1; νAr C–H 3040 cm−1 |





References
- Argun, A.A.; Aubert, P.H.; Thompson, B.C.; Schwendeman, I.; Gaupp, C.L.; Hwang, J.; Pinto, N.J.; Tanner, D.B.; MacDiarmid, A.G.; Reynolds, J.R. Multicolored electrochromism in polymers: Structures and devices. Chem. Mater. 2004, 16, 4401–4412. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in pi-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic Solar Cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for organic transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Wong, J.Y.; Langer, R.; Ingber, D.E. Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.J.; Schell, B.A.; Brady, B.K. Imaging Element Containing Poly(3,4-Ethylene Dioxypyrrole/Styrene Sulfonate). U.S. Patent 5 665 498, 9 September 1997. [Google Scholar]
- Seol, H.; Jeong, H.; Jeon, S. Optoelectrochemical properties of copolymer of terthiophene with 3,4-ethlenedioxypyrrole. J. Electroanal. Chem. 2009, 636, 107–112. [Google Scholar] [CrossRef]
- Chandrasekhar, P.; Zay, B.J.; Birur, G.C.; Rawal, S.; Pierson, E.A.; Kauder, L.; Swanson, T. Large, switchable electrochromism in the visible through far-infrared in conducting polymer devices. Adv. Funct. Mater. 2002, 12, 95–103. [Google Scholar] [CrossRef]
- Zhang, C.; Hua, H.; Liu, J.; Han, X.; Liu, Q.; Wei, Z.; Shao, C.; Hu, C. Enhanced Photocatalytic Activity of Nanoparticle-Aggregated Ag–AgX(X = Cl, Br)@TiO2 Microspheres Under Visible Light. Nano-Micro Lett. 2017, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.L.; Craig, M.R.; Babiarz, J.E.; Kiyak, K.; Reynolds, J.R. Orange and red to transmissive electrochromic polymers based on electron-rich dioxythiophenes. Macromolecules 2010, 43, 4460–4467. [Google Scholar] [CrossRef]
- Chua, L.-L.; Zaumseil, J.; Chang, J.-F.; Ou, E.C.-W.; Ho, P.K.-H.; Sirringhaus, H.; Friend, R.H. General observation of n-type field-effect behaviour in organic semiconductors. Nature 2005, 434, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Li, Y.X.; Yu, X.F.; Yang, Q.B.; Noh, C.H. Using room temperature ionic liquid to fabricate PEDOT/TiO2nanocomposite electrode-based electrochromic devices with enhanced long-term stability. Sol. Energy Mater. Sol. Cells 2008, 92, 1253–1259. [Google Scholar] [CrossRef]
- Tao, Y.J.; Zhou, Y.J.; Xu, X.Q.; Zhang, Z.Y.; Cheng, H.F.; Zheng, W.W. A multielectrochromic copolymer based on anthracene and thiophene via electrochemical copolymerization in boron trifluoride diethyl etherate. Electrochim. Acta 2012, 78, 353–358. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Wang, N.; Xu, Y.; Xiang, W.; Ouyang, M.; Ma, C. Electrosyntheses and characterizations of novel electrochromic copolymers based on pyrene and 3,4-ethylenedioxythiophene. Electrochim. Acta 2009, 55, 13–18. [Google Scholar] [CrossRef]
- Camurlu, P.; Şahmetlioǧlu, E.; Şahin, E.; Akhmedov, I.M.; Tanyeli, C.; Toppare, L. Fine tuning of color via copolymerization and its electrochromic device application. Thin Solid Films 2008, 516, 4139–4144. [Google Scholar] [CrossRef]
- Skotheim, T.A.; Reynolds, J.R. Handbook of Conducting Polymers: Conjugated Polymers Processing and Applications; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420043587. [Google Scholar]
- Oliver, R.; Muñoz, A.; Ocampo, C.; Alemán, C.; Armelin, E.; Estrany, F. Electrochemical characteristics of copolymers electrochemically synthesized from N-methylpyrrole and 3,4-ethylenedioxythiophene on steel electrodes: Comparison with homopolymers. Chem. Phys. 2006, 328, 299–306. [Google Scholar] [CrossRef]
- Jarosz, T.; Gebka, K.; Kepska, K.; Lapkowski, M.; Ledwon, P.; Nitschke, P.; Stolarczyk, A. Investigation of the Effects of Non-Conjugated Co-Grafts on the Spectroelectrochemical and Photovoltaic Properties of Novel Conjugated Graft Copolymers Based on Poly(3-hexylthiophene). Polymers 2018, 10, 1064. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Graft modification of cellulose: Methods, properties and applications. Polymer 2015, 70, A1–A16. [Google Scholar] [CrossRef]
- Abbasi, F.; Mirzadeh, H.; Katbab, A.A. Modification of polysiloxane polymers for biomedical applications: A review. Polym. Int. 2001, 50, 1279–1287. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Ogiwara, N.; Takayanagi, M.; Nagaoka, M.; Kitagawa, S.; Uemura, T. Sequence-regulated copolymerization based on periodic covalent positioning of monomers along one-dimensional nanochannels. Nat. Commun. 2018, 9, 329. [Google Scholar] [CrossRef] [PubMed]
- Belibel, R.; Azzouz, I.; Barbaud, C. Synthesis and characterizations of new isotactic homopolyesters, statistical and block copolyesters derived of poly((S)-3,3-dimethylmalic acid) via the lactone route. J. Polym. Sci. Part A Polym. Chem. 2016, 51, 1495–1507. [Google Scholar] [CrossRef]
- EL-Sukkary, M.M.A.; Ismail, D.A.; Rayes, S.M.E.; Saad, M.A. Synthesis, characterization and surface properties of amino-glycopolysiloxane. J. Ind. Eng. Chem. 2014, 20, 3342–3348. [Google Scholar] [CrossRef]
- Chenthamarakshan, C.R.; Eldo, J.; Ajayaghosh, A. Synthesis and properties of alternating acceptor-donor copolymers of squaric acid with 1-dodecyl- and 3-dodecylpyrroles. Macromolecules 1999, 32, 251–257. [Google Scholar] [CrossRef]
- Ebrahim Attia, A.B.; Ong, Z.Y.; Hedrick, J.L.; Lee, P.P.; Ee, P.L.R.; Hammond, P.T.; Yang, Y.Y. Mixed micelles self-assembled from block copolymers for drug delivery. Curr. Opin. Colloid Interface Sci. 2011, 16, 182–194. [Google Scholar] [CrossRef]
- Patel, S.N.; Javier, A.E.; Beers, K.M.; Pople, J.A.; Ho, V.; Segalman, R.A.; Balsara, N.P. Morphology and thermodynamic properties of a copolymer with an electronically conducting block: Poly(3-ethylhexylthiophene)-block-poly(ethylene oxide). Nano Lett. 2012, 12, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Balverde, S.; Dumur, F.; Lalevée, J.; Poly, J. Synergetic effect of the epoxide functional groups in the photocatalyzed atom transfer radical copolymerization of glycidyl methacrylate. Polym. Chem. 2016, 7, 6084–6093. [Google Scholar] [CrossRef]
- Gu, H.; Ming, S.; Lin, K.; Liu, H.; Chen, S.; Lu, B.; Xu, J. Thermoelectric Properties of Poly(selenophene-co-3, 4-ethylenedioxythiophene) via Electropolymerization. J. Electron. Mater. 2017, 46, 3124–3130. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Liu, J.-W.; Huang, P.-T. A molecular structure and crystallization correlation study of pyromellitic diimide-based conjugated copolymers. J. Chin. Chem. Soc. 2018, 65, 828–834. [Google Scholar] [CrossRef]
- Patel, S.N.; Javier, A.E.; Balsara, N.P. Electrochemically oxidized electronic and ionic conducting nanostructured block copolymers for lithium battery electrodes. ACS Nano 2013, 7, 6056–6068. [Google Scholar] [CrossRef] [PubMed]
- Boudouris, B.W.; Frisbie, C.D.; Hillmyer, M.A. Nanoporous poly(3-alkylthiophene) thin films generated from block copolymer templates. Macromolecules 2008, 41, 67–75. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Fu, Y.; Xu, J.; Nie, G. Electrochromic property of a copolymer based on 5-cyanoindole and 3,4-ethylenedioxythiophene and its application in electrochromic devices. J. Electroanal. Chem. 2013, 700, 17–23. [Google Scholar] [CrossRef]
- Ak, M.; Ak, M.S.; Kurtay, G.; Güllü, M.; Toppare, L. Synthesis and electropolymerization of 1,2-bis(thiophen-3-ylmethoxy)benzene and its electrochromic properties and electrochromic device application. Solid State Sci. 2010, 12, 1199–1204. [Google Scholar] [CrossRef]
- Somani, P.R.; Radhakrishnan, S. Electrochromic Materials and Devices: Present and Future. Mater. Chem. Phys. 2003, 77, 117–133. [Google Scholar] [CrossRef]
- Tao, Y.J.; Zhang, Z.Y.; Xu, X.Q.; Zhou, Y.J.; Cheng, H.F.; Zheng, W.W. Facile and economical synthesis of high-contrast multielectrochromic copolymers based on anthracene and 3,4-ethylenedioxythiophene via electrocopolymerization in boron trifluoride diethyl etherate. Electrochim. Acta 2012, 77, 157–162. [Google Scholar] [CrossRef]
- Nie, G.; Qu, L.; Xu, J.; Zhang, S. Electrosyntheses and characterizations of a new soluble conducting copolymer of 5-cyanoindole and 3,4-ethylenedioxythiophene. Electrochim. Acta 2008, 53, 8351–8358. [Google Scholar] [CrossRef]
- Murotani, A.; Atobe, M.; Fuchigami, T. Electrochemical copolymerization of aromatic compounds in centrifugal fields. J. Electrochem. Soc. 2005, 15, D161–D166. [Google Scholar] [CrossRef]
- Smith, E.L.; Glidle, A.; Mortimer, R.J.; Ryder, K.S. Spectroelectrochemical responses of thin-film conducting copolymers prepared electrochemically from mixtures of 3,4-ethylenedioxythiophene and 2,2′-bithiophene. Phys. Chem. Chem. Phys. 2007, 9, 6098–6105. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Ramachandran, E.; Dhamodharan, R. Conjugated polymers with carbazole, fluorene, and ethylene dioxythiophene in the main chain and a pendant cyano group: Synthesis, photophysical, and electrochemical studies. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2774–2784. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Nie, G.; Han, X.; Zhang, S.; Xu, J.; Cai, T. Electrochemical copolymerization of indole and 3-methylthiophene. J. Appl. Polym. Sci. 2007, 104, 3129–3136. [Google Scholar] [CrossRef]
- Jayakrishnan, K.; Joseph, A.; Bhattathiripad, J.; Ramesan, M.T.; Chandrasekharan, K.; Siji Narendran, N.K. Reverse saturable absorption studies in polymerized indole—Effect of polymerization in the phenomenal enhancement of third order optical nonlinearity. Opt. Mater. (Amst.) 2016, 54, 252–261. [Google Scholar] [CrossRef]
- Wadatkar, N.S.; Waghuley, S.A. Complex optical studies on conducting polyindole as-synthesized through chemical route. Egypt. J. Basic Appl. Sci. 2015, 2, 19–24. [Google Scholar] [CrossRef]
- Holze, R. Copolymers—A refined way to tailor intrinsically conducting polymers. Electrochim. Acta 2011, 56, 10479–10492. [Google Scholar] [CrossRef]
- Billaud, D.; Maarouf, E.B.; Hannecart, E. Electrochemical polymerization of indole. Polymer 1994, 35, 2010–2011. [Google Scholar] [CrossRef]
- Nie, G.; Xu, J.; Zhang, S.; Cai, T.; Han, X. Electrochemical copolymerization of carbazole and 3-methylthiophene. J. Appl. Polym. Sci. 2006, 102, 1877–1885. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, J.; Pu, S.; Du, Y. Electrosyntheses of High-Quality Freestanding Poly(fluorene-co-3-methylthiophene) Films with Tunable Fluorescence Properties. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4904–4915. [Google Scholar] [CrossRef]












| Copolymers/Polymers | Volume Co—Monomers | Concentration [mM] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| In | Cz | Fl | HT | electrolyte | In | Cz | Fl | HT | |
| In-HT 1-10 EP | 0.2 mL | - | - | 1 mL | 0.8 mL | 5 | - | - | 50 |
| In-HT 5-2 EP | 1 mL | - | - | 0.2 mL | 0.8 mL | 25 | - | - | 10 |
| Cz-HT 1-5 EP | - | 0.2 mL | - | 1 mL | 0.8 mL | - | 2 | - | 10 |
| Cz-HT 5-1 EP | - | 1 mL | - | 0.2 mL | 0.8 mL | - | 10 | - | 2 |
| Fl-HT 1-5 EP | - | - | 0.2 mL | 1 mL | 0.8 mL | - | - | 2 | 10 |
| Fl-HT 5-1 EP | - | - | 1 mL | 0.2 mL | 0.8 mL | - | - | 10 | 2 |
| In EP | 2 mL | - | - | - | - | 50 | - | - | - |
| Cz EP | - | 2 mL | - | - | - | 20 | - | - | - |
| HT EP | - | - | - | 2 mL | - | - | - | - | 100 |
| Fl Ep | - | - | 2 mL | - | - | - | - | 40 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebka, K.; Jarosz, T.; Stolarczyk, A. The Different Outcomes of Electrochemical Copolymerisation: 3-Hexylthiophene with Indole, Carbazole or Fluorene. Polymers 2019, 11, 355. https://doi.org/10.3390/polym11020355
Gebka K, Jarosz T, Stolarczyk A. The Different Outcomes of Electrochemical Copolymerisation: 3-Hexylthiophene with Indole, Carbazole or Fluorene. Polymers. 2019; 11(2):355. https://doi.org/10.3390/polym11020355
Chicago/Turabian StyleGebka, Karolina, Tomasz Jarosz, and Agnieszka Stolarczyk. 2019. "The Different Outcomes of Electrochemical Copolymerisation: 3-Hexylthiophene with Indole, Carbazole or Fluorene" Polymers 11, no. 2: 355. https://doi.org/10.3390/polym11020355
APA StyleGebka, K., Jarosz, T., & Stolarczyk, A. (2019). The Different Outcomes of Electrochemical Copolymerisation: 3-Hexylthiophene with Indole, Carbazole or Fluorene. Polymers, 11(2), 355. https://doi.org/10.3390/polym11020355