Delayed Crosslinking Amphiphilic Polymer Gel System with Adjustable Gelation Time Based on Competitive Inclusion Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrophobically Modified Polyacrylamide
2.3. Rheology Measurement of Polymer Solutions and Polymer Gel Systems
2.4. Intrinsic Viscosity Measurement and Molecular Weight Estimation for Polymer
2.5. Preparation of β-CD/Crosslinker Inclusion Complex
2.6. Determination of Crosslinker Concentration during Controlled Released Process
2.7. Measurement of Gelation Time and Gel Strength
3. Results and Discussions
3.1. Solution Properties of P(AM–NaA–DDAM)
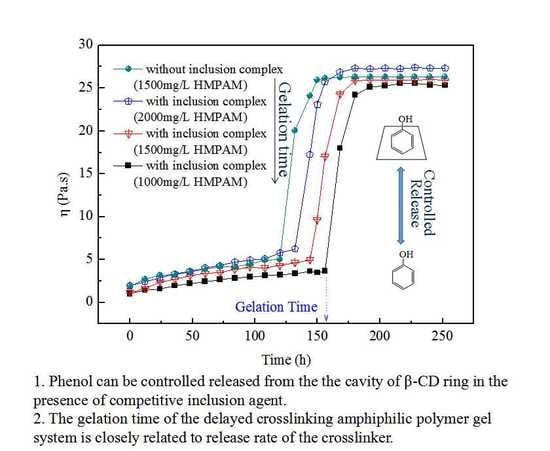
3.2. Gelling Properties of the Delayed Crosslinking HMPAM Gel System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.Q.; Zhang, H.L.; Wang, S.; Wang, H.; Bao, S.H. Research on mechanisms of steam breakthrough and profile control design for steam soaking well. Pet. Sci. 2006, 3, 51–55. [Google Scholar]
- Hua, Z.; Lin, M.; Guo, J.; Xu, F.; Li, Z.; Li, M. Study on plugging performance of cross-linked polymer microspheres with reservoir pores. J. Pet. Sci. Eng. 2013, 105, 70–75. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, G.; Hua, Z.; Zhao, Q.; Sun, F. Conformation and plugging properties of crosslinked polymer microspheres for profile control. Colloid Surface A 2015, 477, 49–54. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, C.; Chen, A.; Yan, Z.; Zhao, M. Experimental study and application of gels formed by nonionic polyacrylamide and phenolic resin for in-depth profile control. J. Pet. Sci. Eng. 2015, 135, 552–560. [Google Scholar] [CrossRef]
- Vossoughi, S. Profile modification using in situ gelation technology—A review. J. Pet. Sci. Eng. 2000, 26, 199–209. [Google Scholar] [CrossRef]
- Sun, F.; Lin, M.; Dong, Z.; Zhu, D.; Wang, S.L.; Yang, J. Effect of composition of HPAM/chromium(iii) acetate gels on delayed gelation time. J. Dispers. Sci. Technol. 2016, 37, 753–759. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, L.; Pu, J.; Pu, C.; Cui, S. Influence of HPAM molecular weight on the cross-linking reaction of HPAM/Cr3+ and transportation of HPAM/Cr3+ in micro-fractures. Energy Fuel 2016, 30, 9351–9361. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Wang, R.; Liu, Y.; Zhang, S. Study of action mechanisms and properties of Cr3+ cross-linked polymer solution with high salinity. Petrol. Sci. 2012, 9, 75–81. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Ge, J.; Jiang, P.; Zhu, X. Experimental research of syneresis mechanism of HPAM/Cr3+ gel. Colloid Surface A 2015, 483, 96–103. [Google Scholar] [CrossRef]
- Bassett, D.C.; Håti, A.G.; Melø, T.B.; Stokke, B.T.; Sikorski, P. Competitive ligand exchange of crosslinking ions for ionotropic hydrogel formation. J. Mater. Chem. B 2016, 4, 6175–6182. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Pu, W.; Zhao, J.; Liao, R. Experimental investigation of the novel phenol-formaldehyde cross-linking HPAM gel system: Based on the secondary cross-linking method of organic cross-linkers and its gelation performance study after flowing through porous media. Energy Fuel 2011, 25, 727–736. [Google Scholar] [CrossRef]
- Gu, C.; Lv, Y.; Fan, X.; Zhao, C.; Dai, C.; Zhao, G. Study on rheology and microstructure of phenolic resin cross-linked nonionic polyacrylamide (NPAM) gel for profile control and water shutoff treatments. J. Pet. Sci. Eng. 2018, 169, 546–552. [Google Scholar] [CrossRef]
- Wei, F.; Liu, Y.; Yue, X.; Hou, J.; Tang, X.; Li, Y.; Xiong, C.; Liu, G. Thermal stability and transport property of multiple emulsions used as delayed crosslinker. Acta Petrol. Sin. 2008, 29, 423–426. [Google Scholar]
- Xu, B.; Zhang, J.; Hu, X. The Delayed Crosslinking Amphiphilic Polymer Gel System Based on Multiple Emulsion for in-Depth Profile Control. J. Dispers. Sci. Technol. 2017, 38, 1242–1246. [Google Scholar] [CrossRef]
- Yang, M.H. The rheological behavior of polyacrylamide solution. J. Polym. Eng. 1999, 19, 371–381. [Google Scholar] [CrossRef]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Characterization of acrylamide polymers for enhanced oil recovery. J. Appl. Polym. Sci. 2003, 87, 1869–1878. [Google Scholar] [CrossRef]
- Vinu, R.; Madras, G. Photocatalytic degradation of poly(acrylamide-co-acrylic acid). J. Phys. Chem. B 2008, 112, 8928–8935. [Google Scholar] [CrossRef]
- Abhijit, S.; Achinta, B.; Keka, O.; Ajay, M. Effects of alkali, salts, and surfactant on rheological behaviour of partially hydrolysed polyacrylamide solutions. J. Chem. Eng. Data 2010, 55, 4315–4322. [Google Scholar]
- Hoefner, M.L.; Seetharam, R.V.; Shu, P.; Phelps, C.H. Selective penetration of biopolymer profile-control gels: Experiment and model. J. Pet. Sci. Eng. 1992, 7, 53–66. [Google Scholar] [CrossRef]
- Taylor, K.C.; NasrElDin, A. Water-soluble hydrophobically associating polymers for improved oil recovery: A literature review. J. Pet. Sci. Eng. 1998, 192, 265–280. [Google Scholar] [CrossRef]
- Dastan, S.; Hassnajili, S.; Abdollahi, E. Hydrophobically associating terpolymers of acrylamide, alkyl acrylamide, and methacrylic acid as EOR thickeners. J. Polym. Res. 2016, 23, 175. [Google Scholar] [CrossRef]
- Lai, N.; Dong, W.; Ye, Z.; Dong, J.; Qin, X.; Chen, W.; Ke, C. A water-soluble acrylamide hydrophobically associating polymer: Synthesis, characterization, and properties as EOR chemical. J. Appl. Polym. Sci. 2013, 129, 1888–1896. [Google Scholar] [CrossRef]
- Zhuang, D.Q.; Da, A.H.; Zhang, Y.X.; Dieing, R.; Ma, L.; Haeussling, L. Hydrophobically modified polyelectrolytes II: Synthesis and characterization of poly(acrylic acid-co-alkylacrylate). Polym. Adv. Technol. 2001, 12, 616–625. [Google Scholar] [CrossRef]
- McCormick, C.L.; Nonaka, T.; Johnson, C.B. Water-soluble copolymers: 27. Synthesis and aqueous solution behavior of associative acrylamide/N-alkylacrylamide copolymers. Polymer 1988, 29, 731–739. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Chassenieux, C.; Nicolai, T.; Benyahia, L. Rheology of associative polymer solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 18–26. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, W.; Yang, M. Synthesis and solution properties of an associative polymer with excellent salt-thickening. J. Appl. Polym. Sci. 2012, 125, 4049–4059. [Google Scholar] [CrossRef]
- Zhong, C.; Jiang, L.; Peng, X. Synthesis and solution behavior of comb-like terpolymers with poly(ethylene oxide) macromonomer. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1241–1250. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Chen, W.; Yu, H.; Qi, Z.; Li, K. Preparation and solution characteristics of a novel hydrophobically associating terpolymer for enhanced oil recovery. J. Solut. Chem. 2011, 40, 447–457. [Google Scholar] [CrossRef]
- Fagui, A.E.; Dalmas, F.; Lorthioir, C.; Wintgens, V.; Volet, G.; Amiel, C. Well-defined core-shell nanoparticles containing cyclodextrin in the shell: A comprehensive study. Polymer 2011, 52, 3752–3761. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M. Polymeric self-assembly into micelles and hollow spheres with multiscale cavities driven by inclusion complexation. J. Am. Chem. Soc. 2006, 128, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sun, X.Y.; Zhou, Y.F.; Yan, D.Y. Supramolecular self-assembly and controllable drug release of thermosensitive hyperbranched multiarm copolymers. Sci. China Chem. 2010, 53, 487–494. [Google Scholar] [CrossRef]
- Guo, X.; Abdala, A.A.; May, B.L.; Lincoln, S.F.; Khan, S.A.; Prud’Homme, R.K. Novel associative polymer networks based on cyclodextrin inclusion compounds. Macromolecules 2005, 38, 3037–3040. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sá Couto, A.R.; Ryzhakov, A.; Loftsson, T. Self-assembly of α-cyclodextrin and β-cyclodextrin: Identification and Development of Analytical Techniques. J. Pharm. Sci. 2018, 107, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.D.; Ahsan, F. Influence of surfactants (present in the dissolution media) on the release behaviour of tolbutamide from its inclusion complex with β-cyclodextrin. Eur. J. Pharm. Sci. 2000, 9, 291–299. [Google Scholar] [CrossRef]
- Islam, M.F.; Jenkins, R.D.; Bassett, D.R.; Lau, W.; Ou-Yang, H.D. Single chain characterization of hydrophobically modified polyelectrolytes using cyclodextrin/hydrophobe complexes. Macromolecules 2000, 33, 2480–2485. [Google Scholar] [CrossRef]
- Dai, S.; Tam, K.C.; Jenkins, R.D. Microstructure of dilute hydrophobically modified alkali soluble emulsion in aqueous salt solution. Macromolecules 2000, 33, 404–411. [Google Scholar] [CrossRef]
- Nguyen, N.T.B.; Tu, T.N.; Bae, W.; Dang, C.T.Q.; Chung, T.; Nguyen, H.X. Gelation time optimization for an HPAM/chromium acetate system: The successful key of conformance control technology. Energy Source Part A 2012, 34, 1305–1317. [Google Scholar] [CrossRef]
- Moradiaraghi, A. A review of thermally stable gels for fluid diversion in petroleum production. J. Pet. Sci. Eng. 2000, 26, 1–10. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Zhang, H.; Bian, H. Delayed Crosslinking Amphiphilic Polymer Gel System with Adjustable Gelation Time Based on Competitive Inclusion Method. Polymers 2019, 11, 381. https://doi.org/10.3390/polym11020381
Xu B, Zhang H, Bian H. Delayed Crosslinking Amphiphilic Polymer Gel System with Adjustable Gelation Time Based on Competitive Inclusion Method. Polymers. 2019; 11(2):381. https://doi.org/10.3390/polym11020381
Chicago/Turabian StyleXu, Bin, Huiming Zhang, and He Bian. 2019. "Delayed Crosslinking Amphiphilic Polymer Gel System with Adjustable Gelation Time Based on Competitive Inclusion Method" Polymers 11, no. 2: 381. https://doi.org/10.3390/polym11020381
APA StyleXu, B., Zhang, H., & Bian, H. (2019). Delayed Crosslinking Amphiphilic Polymer Gel System with Adjustable Gelation Time Based on Competitive Inclusion Method. Polymers, 11(2), 381. https://doi.org/10.3390/polym11020381