Investigation of the Structure-Property Effect of Phosphorus-Containing Polysulfone on Decomposition and Flame Retardant Epoxy Resin Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of EP/Polysulfone Composites
2.3. Measurements and Characterization
3. Results and Discussion
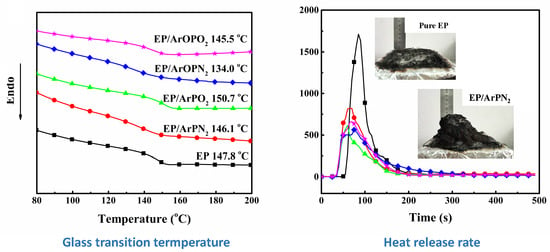
3.1. Thermal Behaviors
3.2. Flammability
3.3. Gas Phase Mechanisms
3.4. Condensed Phase Mechanisms
3.5. Thermal Degradation Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pomázi, Á.; Toldy, A. Particle distribution of solid flame retardants in infusion moulded composites. Polymers 2017, 9, 250. [Google Scholar] [CrossRef]
- Zhao, X.M.; Zhang, L.; Alonso, J.P.; Delgado, S.; Martínez-Miranda, M.R.; Wang, D.Y. Influence of phenylphosphonic amide on rheological, mechanical and flammable properties of carbon fiber/RTM6 composites. Compos. Part B 2017, 149, 74–81. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Pomázi, Á.; Szolnoki, B.; Toldy, A. Flame retardancy of low-viscosity epoxy resins and their carbon fibre reinforced composites via a combined solid and gas phase mechanism. Polymers 2018, 10, 1081. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.; Altstädt, V.; et al. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An overview of mode of action and analytical methods for evaluation of gas phase activities of flame retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Täuber, K.; Marsico, F.; Wurm, F.R.; Schartel, B. Hyperbranched poly(phosphoester)s as flame retardants for technical and high performance polymers. Polym. Chem. 2014, 5, 7042–7053. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.P.; Zhang, Y.; Ban, D.M. Simple green synthesis of solid polymeric bisphenol A bis(diphenyl phosphate) and its flame retardancy in epoxy resins. RSC Adv. 2015, 5, 80415–80423. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zheng, C.Y.; Yang, K.K. Synthesis and characterization of polysulfonyl diphenylene phenyl phosphonate. Polym. Mat. Sci. Eng. 1999, 15, 53–56. [Google Scholar]
- Zhao, W.; Wang, G.; Liu, J.P.; Ban, D.M.; Cheng, T.J.; Liu, X.D.; Li, Y.X.; Huang, Y.Q. Synthesis, structure–property and flame retardancy relationships of polyphosphonamide and its application on epoxy resins. RSC Adv. 2017, 7, 49863–49874. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wu, W.H.; Wang, Y.H.; Jiao, Y.H.; Lu, L.Y.; Qu, H.Q.; Qin, X.Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.M.; Sandler, J.K.W.; Altstädt, V.; Hoffmann, T.; Pospiech, D.; Ciesielski, M.; Döring, M.; Braun, U.; Balabanovich, A.I.; Schartel, B. Novel phosphorus-modified polysulfone as a combined flame retardant and toughness modifier for epoxy resins. Polymer 2007, 48, 778–790. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, J.C.; Huang, C.M.; Jehng, J.M.; Chang, H.C.; Juang, T.Y.; Su, W.C. Side-chain phenol-functionalized poly(ether sulfone) and its contribution to high-performance and flexible epoxy thermosets. Polymer 2013, 54, 6936–6941. [Google Scholar] [CrossRef]
- Liaw, D.J.; Shen, W.C. Synthesis of aromatic polyphosphonate: low temperature solution polycondensation of 4,4′-sulphonyldiphenol with phenoxy dichlorophosphate. Polymer 1993, 34, 1336–1338. [Google Scholar] [CrossRef]
- Tai, Q.L.; Hu, Y.; Yuen, R.K.K.; Song, L.; Lu, H.D. Synthesis, structure-property relationships of polyphosphoramides with high char residues. J. Mater. Chem. A. 2011, 21, 6621–6627. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.P.; Peng, H.; Liao, J.Y.; Wang, X.J. Synthesis of a novel PEPA-substituted polyphosphoramide with high char residues and its performance as an intumescent flame retardant for epoxy resins. Polym. Degrad. Stab. 2015, 118, 120–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, J.; Peng, H.; Qu, H.Q.; Hao, J.W. Catalytic pyrolysis and flame retardancy of epoxy resins with solid acid boron phosphate. Polym. Degrad. Stab. 2014, 110, 395–404. [Google Scholar] [CrossRef]
- Tian, N.N.; Gong, J.; Wen, X.; Yao, K.; Tang, T. Synergistic effect between a novel char forming agent and ammonium polyphosphate on flame retardancy and thermal properties of polypropylene. RSC Adv. 2013, 52, 10905–10915. [Google Scholar] [CrossRef]








| Samples | DGEBA (g) | m-PDA (g) | Polysulfone (g) | P (wt %) | UL-94 (3.2 mm) |
|---|---|---|---|---|---|
| EP | 50.00 | 6.00 | 0 | 0 | No rating |
| EP/ArPN2 | 50.00 | 6.00 | 9.88 | 1.25 | V-0 |
| EP/ArPO2 | 50.00 | 6.00 | 9.88 | 1.25 | V-1 |
| EP/ArOPN2 | 50.00 | 6.00 | 10.35 | 1.25 | No rating |
| EP/ArOPO2 | 50.00 | 6.00 | 10.35 | 1.25 | No rating |
| Sample | Tg (°C) | Tonset (°C) | Tmax (°C) | Char a Exp./Calcd. |
|---|---|---|---|---|
| EP | 147.8 | 369 | 394 | 12.7/-- |
| EP/ArPN2 | 146.1 | 317 | 339,373 | 21.7/18.0 |
| EP/ArPO2 | 150.7 | 317 | 364 | 24.9/16.4 |
| EP/ArOPN2 | 134.0 | 313 | 365 | 25.2/17.5 |
| EP/ArOPO2 | 145.5 | 310 | 365 | 27.5/17.0 |
| Sample | TTI (s) | PHRR (kW·m−2) | THR (MJ·m−2) | TML (%) | TSR (m2) | THR/TML (MJ·m−2·g−1) | TSR/TML (g−1) | TCOR/TML (g·g−1) |
|---|---|---|---|---|---|---|---|---|
| EP | 50 | 1712 | 83.7 | 93.0 | 22.4 | 3.85 | 117 | 0.11 |
| EP/ArPN2 | 29 | 847 | 61.5 | 80.2 | 18.3 | 3.27 | 110 | 0.15 |
| EP/ArPO2 | 32 | 608 | 42.7 | 82.7 | 19.2 | 2.31 | 117 | 0.18 |
| EP/ArOPN2 | 30 | 546 | 59.4 | 77.1 | 11.4 | 3.42 | 75 | 0.14 |
| EP/ArOPO2 | 30 | 726 | 55.3 | 80.8 | 14.8 | 3.09 | 94 | 0.14 |
| Sample | C (% a) | N (%) | O (%) | P (%) | S (%) |
|---|---|---|---|---|---|
| EP | 82.82 | 3.22 | 13.77 | 0.10 | 0.09 |
| EP/ArPN2 Inner | 82.49 | 1.10 | 16.10 | 0.13 | 0.18 |
| EP/ArPN2 Outer | 79.19 | 3.16 | 16.65 | 0.84 | 0.17 |
| EP/ArPO2 Inner | 81.80 | 1.11 | 16.74 | 0.14 | 0.21 |
| EP/ArPO2 Outer | 75.52 | 2.88 | 19.28 | 2.05 | 0.26 |
| EP/ArOPN2 Inner | 80.87 | 1.09 | 17.52 | 0.28 | 0.24 |
| EP/ArOPN2 Outer | 71.34 | 5.53 | 19.78 | 2.92 | 0.43 |
| EP/ArOPO2 Inner | 80.92 | 3.26 | 14.35 | 1.24 | 0.23 |
| EP/ArOPO2 Outer | 72.17 | 4.50 | 19.98 | 2.87 | 0.48 |
| α | EP | EP/ArPN2 | EP/ArPO2 | EP/ArOPN2 | EP/ArOPO2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ea (kJ/mol) | r | Ea (kJ/mol) | r | Ea (kJ/mol) | r | Ea (kJ/mol) | r | Ea (kJ/mol) | r | |
| 0.10 | 147.37 | 0.9967 | 147.37 | 0.9967 | 138.74 | 0.9716 | 140.61 | 0.9816 | 99.57 | 0.9145 |
| 0.20 | 147.37 | 0.9967 | 147.37 | 0.9967 | 167.26 | 0.9593 | 167.26 | 0.9593 | 123.07 | 0.9210 |
| 0.30 | 147.37 | 0.9967 | 135.22 | 0.9907 | 168.51 | 0.9884 | 187.00 | 0.9665 | 136.91 | 0.9220 |
| 0.40 | 160.52 | 0.9974 | 136.91 | 1.0000 | 168.51 | 0.9884 | 167.26 | 0.9593 | 148.01 | 0.9586 |
| 0.50 | 165.05 | 0.9959 | 147.37 | 0.9967 | 168.51 | 0.9884 | 167.26 | 0.9593 | 148.74 | 0.9189 |
| 0.60 | 165.05 | 0.9959 | 147.37 | 0.9967 | 185.75 | 0.9876 | 185.75 | 0.9876 | 148.74 | 0.9189 |
| 0.70 | 165.05 | 0.9959 | 165.05 | 0.9959 | 187.00 | 0.9665 | 209.03 | 0.9650 | 169.86 | 0.9215 |
| 0.80 | 165.05 | 0.9959 | 209.40 | 0.9956 | 240.97 | 0.9757 | 240.97 | 0.9757 | 209.03 | 0.9650 |
| 0.85 | 167.34 | 0.9949 | 221.17 | 0.9962 | 243.09 | 0.9370 | 296.63 | 0.9657 | 283.60 | 0.9604 |
| 0.90 | 178.58 | 0.9836 | 260.79 | 0.9636 | 250.73 | 0.8971 | 406.83 | 0.9740 | 383.36 | 0.9849 |
| 0.95 | 383.36 | 0.9849 | 315.60 | 0.9846 | 312.94 | 0.9335 | 410.74 | 0.9220 | 406.83 | 0.9740 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Li, Y.; Li, Q.; Wang, Y.; Wang, G. Investigation of the Structure-Property Effect of Phosphorus-Containing Polysulfone on Decomposition and Flame Retardant Epoxy Resin Composites. Polymers 2019, 11, 380. https://doi.org/10.3390/polym11020380
Zhao W, Li Y, Li Q, Wang Y, Wang G. Investigation of the Structure-Property Effect of Phosphorus-Containing Polysulfone on Decomposition and Flame Retardant Epoxy Resin Composites. Polymers. 2019; 11(2):380. https://doi.org/10.3390/polym11020380
Chicago/Turabian StyleZhao, Wei, Yongxiang Li, Qiushi Li, Yiliang Wang, and Gong Wang. 2019. "Investigation of the Structure-Property Effect of Phosphorus-Containing Polysulfone on Decomposition and Flame Retardant Epoxy Resin Composites" Polymers 11, no. 2: 380. https://doi.org/10.3390/polym11020380
APA StyleZhao, W., Li, Y., Li, Q., Wang, Y., & Wang, G. (2019). Investigation of the Structure-Property Effect of Phosphorus-Containing Polysulfone on Decomposition and Flame Retardant Epoxy Resin Composites. Polymers, 11(2), 380. https://doi.org/10.3390/polym11020380