Preparation and Characterization of Transparent Polyimide–Silica Composite Films Using Polyimide with Carboxylic Acid Groups
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Poly(amic acid)
2.2. Preparation of PI–Silica Composite Films.
2.3. Measurements.
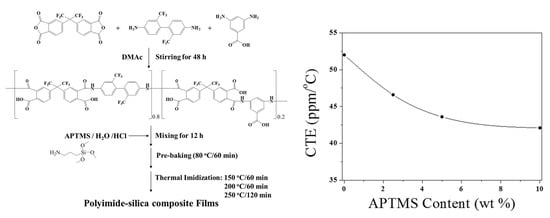
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lewis, J. Material challenge for flexible organic devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Charton, C.; Schiller, N.; Fahland, M.; Hollander, A.; Wedel, A.; Noller, K. Development of high barrier films on flexible polymer substrates. Thin Solid Films 2006, 502, 99–103. [Google Scholar] [CrossRef]
- Burrows, P.E.; Graff, G.L.; Gross, M.E.; Martin, P.M.; Shi, M.K.; Hall, M.; Mast, E.; Bonham, C.; Bennett, W.; Sullivanb, M.B. Ultra barrier flexible substrates for flat panel displays. Displays 2001, 22, 65–69. [Google Scholar] [CrossRef]
- Ha, Y.; Choi, M.-C.; Jo, N.; Ha, C.-S.; Han, D.; Hams, S.; Han, M. Polyimide multilayer thin films prepared via spin coating from poly(amic acid) and poly(amic acid) ammonium salt. Macromol. Res. 2008, 16, 725–733. [Google Scholar] [CrossRef]
- Ree, M. High performance polyimides for applications in microelectronics and flat panel displays. Macromol. Res. 2006, 14, 1–33. [Google Scholar] [CrossRef]
- Seo, H.; Chae, B.; Im, J.H.; Jung, Y.M.; Lee, S.W. Imidization induced structural changes of 6FDA-ODA poly(amic acid) by two-dimensional (2D) infrared correlation spectroscopy. J. Mol. Struct. 2014, 1069, 196–199. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M. Synthesis and characterization of aromatic–aliphatic polyamide nanocomposite films incorporating a thermally stable organoclay. Nanoscale Res. Lett. 2009, 4, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Harada, M.; Okada, T.; Ishige, R. Effective reduction of volumetric thermal expansion of aromatic polyimide films by incorporating interchain crosslinking. Polymers 2018, 10, 761. [Google Scholar] [CrossRef]
- Yeo, H.; Goh, M.; Ku, B.-C.; You, N.-H. Synthesis and characterization of highly-fluorinated colorless polyimides derived from 4,4′-((perfluoro-[1,1′-biphenyl]-4,4′-diyl)bis(oxy))bis(2,6-dimethylaniline) and aromatic dianhydrides. Polymer 2015, 76, 280–286. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Yen, H.-J.; Liou, G.-S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Choi, M.C.; Nagappan, S.; Ha, C.S. Synthesis and characterization of highly transparent and hydrophobic fluorinated polyimides derived from perfluorodecylthio substituted diamine monomers. J. Polym. Sci. A Polym. Chem. 2015, 53, 479–488. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Chang, F.-C. Highly organosoluble and flexible polyimides with color lightness and transparency based on 2,2-bis[4-(2-trifluoromethyl-4-aminophenoxy)-3,5-dimethylphenyl]propane. J. Polym. Sci. A Polym. Chem. 2004, 42, 5766–5774. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Yang, C.-P.; Chu, K.-Y. Synthesis and properties of poly(ether imide)s having ortho-linked aromatic units in the main chain. Macromolecules 1997, 30, 165–170. [Google Scholar] [CrossRef]
- Volksen, W.; Cha, H.J.; Sanchez, M.I.; Yoon, D.Y. Polyimides derived from nonaromatic monomers: Synthesis, characterization and potential applications. React. Funct. Polym. 1996, 30, 61–69. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Yang, C.-P.; Chen, S.-H. Synthesis and properties of ortho-linked polyamides based on a bis(ether-carboxylic acid) or a bis(ether amine) derived from 4-tert-butylcatechol. Polymer 2000, 41, 6537–6551. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Fan, L.; Yang, S. Synthesis and characterization of novel fluorinated polyimides derived from 4,4′-[2,2,2-trifluoro-1-(3,5-ditrifluoromethylphenyl)ethylidene]diphthalic anhydride and aromatic diamines. Polymer 2006, 47, 1443–1450. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.-P. Optical properties of functional hybrid organic–inorganic nanocomposites. Adv. Funct. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Chen, C.-J.; Tsai, C.-L.; Liou, G.-S. Electrically programmable digital memory behaviors based on novel functional aromatic polyimide/TiO2 hybrids with a high ON/OFF ratio. J. Mater. Chem. C 2014, 2, 2842–2850. [Google Scholar] [CrossRef]
- Liou, G.-S.; Lin, P.-H.; Yen, H.-J.; Yu, Y.; Tsai, T.-W.; Chen, W.-C. Highly flexible and optical transparent 6F-PI/TiO2 optical hybrid films with tunable refractive index and excellent thermal stability. J. Mater. Chem. 2010, 20, 531–536. [Google Scholar] [CrossRef]
- Laine, R.M. Nanobuilding blocks based on the [OSiO1.5]x (x = 6, 8, 10) octasilsesquioxanes. J. Mater. Chem. 2005, 15, 3725–3744. [Google Scholar] [CrossRef]
- Pereira, F.; Valle, K.; Bellevlle, P.; Morin, A.; Lambert, S.; Sanchez, C. Advanced mesostructured hybrid silica−nafion membranes for high-performance PEM fuel cell. Chem. Mater. 2008, 20, 1710–1718. [Google Scholar] [CrossRef]
- Zhou, Z.; Franz, A.W.; Hartmann, M.; Seifert, A.; Muller, T.J.J.; Thiel, W.R. Novel organic/inorganic hybrid materials by covalent anchoring of phenothiazines on MCM-41. Chem. Mater. 2008, 20, 4986–4992. [Google Scholar] [CrossRef]
- Sanchez, C.; Soler-Illia, G.J.A.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed hybrid organic-inorganic nanocomposites from functional nanobuilding blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Saba, N.; Tahir, P.M.; Jawaid, M. A review on potentiality of nano filler/natural fiber filled polymer hybrid composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Bellevlle, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2015, 15, 3559–3592. [Google Scholar] [CrossRef]
- Huang, H.-S.; Wilkes, G.L.; Carlson, J.G. Structure-property behaviour of hybrid materials incorporating tetraethoxysilane with multifunctional poly(tetramethylene oxide). Polymer 1989, 30, 2011–2012. [Google Scholar] [CrossRef]
- Huang, H.-S.; Wilkes, G.L. Structure-property behavior of new hybrid materials incorporating oligomeric poly(tetramethylene oxide) with inorganic silicates by a sol-gel process. Polym. Bull. 1987, 18, 455–462. [Google Scholar] [CrossRef]
- Mark, J.E.; Pan, S.-J. Reinforcement of polydimethylsiloxane networks by in situ precipitation of silica: A new method for preparation of filled elastomers. Makromol. Chem. Rapid Commun. 1982, 3, 681–685. [Google Scholar] [CrossRef]
- Mark, J.E.; Jiang, C.-Y.; Tang, M.-Y. Simultaneous Curing and Filling of Elastomers. Macromolecules 1984, 17, 2613–2616. [Google Scholar] [CrossRef]
- Chen, Y.; Iroh, J.O. Synthesis and characterization of polyimide/silica hybrid composites. Chem. Mater. 1999, 11, 1218–1222. [Google Scholar] [CrossRef]
- Shang, Z.; Lu, C.; Gao, L. Preparation and properties of ternary polyimide/SiO2/polydiphenylsiloxane composite films. Polym. Int. 2006, 55, 1277–1282. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Huang, S.-L.; Lin, J.C.M.; Ko, C.-J.; Chen, Y.-L.; Lu, C.-M.; Chen, C.-J. Synthesis and properties of hydrogen-bonded poly(amide-imide) hybrid films. J. Appl. Polym. Sci. 2007, 105, 3689–3697. [Google Scholar] [CrossRef]
- Yen, C.-T.; Chen, W.-C.; Liaw, D.-J.; Lu, H.-Y. Synthesis and properties of new polyimide–silica hybrid films through both intrachain and interchain bonding. Polymer 2003, 44, 7079–7087. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, E.; Kwon, Y.S.; Cho, W.J.; Cho, C.; Chang, M.; Ree, M.; Ha, C.-S. Electrical properties of silica-polyimide composite dielectric thin films prepared via sol-gel reaction and thermal imidization. Synth. Met. 1997, 85, 1399–1400. [Google Scholar] [CrossRef]
- Kwon, S.J.; Jang, A.-R.; Bae, J.; Kim, Y.S.; Lee, S.W. Preparation and characterization of transparent polyimide composite films for flexible substrate. J. Nanoelectron. Optoelectron. 2013, 8, 588–593. [Google Scholar] [CrossRef]
- Kwon, S.J.; Hwang, C.H.; Jang, A.-R.; Bae, J.; Chae, B.; Won, J.C.; Lee, S.W. Preparation and Characterization of Transparent Polyimide/Silica Composite. Mol. Cryst. Liq. Cryst. 2013, 584, 9–17. [Google Scholar] [CrossRef]
- Buffeteau, T.; Labarthet, F.L.; Pezole, M.; Sourisseau, C. Dynamics of photoinduced orientation of nonpolar azobenzene groups in polymer films. Characterization of the cis isomers by visible and FTIR spectroscopies. Macromolecules 2001, 34, 7514–7521. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.I.; Lee, B.; Choi, W.; Chae, B.; Kim, S.B.; Ree, M. Photoreactions and photoinduced molecular orientations of films of a photoreactive polyimide and their alignment of liquid crystals. Macromolecules 2003, 36, 6527–6536. [Google Scholar] [CrossRef]
- Chae, B.; Kim, S.B.; Lee, S.W.; Kim, S.I.; Choi, W.; Lee, B.; Ree, M.; Lee, K.H.; Jung, J.C. Surface morphology, molecular reorientation, and liquid crystal alignment properties of rubbed nanofilms of a well-defined brush polyimide with a fully rodlike backbone. Macromolecules 2002, 35, 10119–10130. [Google Scholar] [CrossRef]
- Lee, S.W.; Chae, B.; Lee, B.; Choi, W.; Kim, S.B.; Kim, S.I.; Park, S.-M.; Jung, J.C.; Lee, K.H.; Ree, M. Rubbing-induced surface morphology and polymer segmental reorientations of a model rrush polyimide and interactions with liquid crystals at the surface. Chem. Mater. 2003, 15, 3105–3112. [Google Scholar] [CrossRef]
- Shin, T.; Ree, M. In situ infrared spectroscopy study on imidization reaction and imidization-induced refractive index and thickness variations in microscale thin films of a poly(amic ester). Langmuir 2015, 21, 6081–6085. [Google Scholar] [CrossRef] [PubMed]
- Boroglu, M.S.; Gurkaynak, A. The preparation of novel silica modified polyimide membranes: Synthesis, characterization, and gas separation properties. Polym. Adv. Technol. 2011, 22, 545–553. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Whang, W.-T. Dynamic mechanical properties of polyimide/poly(silsesquioxane)-like hybrid films. J. Appl. Polym. Sci. 2001, 81, 2500–2516. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Okrasa, L.; Kalvachev, Y. The effect of zeolite L content on dielectric behaviour and thermal stability of polyimide thin films. J. Mater. Sci. 2012, 47, 6354–6365. [Google Scholar] [CrossRef]
- Huertas, R.M.; Maya, E.M.; Abajo, J.; Campa, J.G. Effect of 3,5-diaminobenzoic acid content, casting solvent, and physical aging on gas permeation properties of copolyimides containing pendant acid groups. Macromol. Res. 2011, 19, 797–808. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.M.; Park, H.B.; Lee, Y.M. The gas separation properties of carbon molecular sieve membranes derived from polyimides having carboxylic acid groups. J. Membr. Sci. 2004, 235, 139–146. [Google Scholar] [CrossRef]




| Sample Designation | Feed Content of APTMS a | Tg (°C) b | Td (°C) c | RW (%) d | Remarks e |
|---|---|---|---|---|---|
| PI | - | - f | 364 | 46.2 | T |
| PI–silica-2.5 | 2.5 | - | 393 | 49.2 | T |
| PI–silica-5.0 | 5.0 | - | 510 | 53.0 | T |
| PI–silica-10.0 | 10.0 | - | 497 | 58.2 | T |
| Sample Designation | Film Thickness (µm) | Yellowness Index a | Haze (%) | CTE b |
|---|---|---|---|---|
| PI | 105.7 | 21.3 | 0.83 | 52.0 |
| PI–silica-2.5 | 104.3 | 35.2 | 1.14 | 46.6 |
| PI–silica-5.0 | 97.9 | 40.1 | 1.20 | 43.6 |
| PI–silica-10.0 | 99.7 | 51.8 | 1.35 | 42.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, K.H.; Chae, B.; Kim, K.S.; Lee, S.W.; Jung, Y.M. Preparation and Characterization of Transparent Polyimide–Silica Composite Films Using Polyimide with Carboxylic Acid Groups. Polymers 2019, 11, 489. https://doi.org/10.3390/polym11030489
Moon KH, Chae B, Kim KS, Lee SW, Jung YM. Preparation and Characterization of Transparent Polyimide–Silica Composite Films Using Polyimide with Carboxylic Acid Groups. Polymers. 2019; 11(3):489. https://doi.org/10.3390/polym11030489
Chicago/Turabian StyleMoon, Kwan Ho, Boknam Chae, Ki Seung Kim, Seung Woo Lee, and Young Mee Jung. 2019. "Preparation and Characterization of Transparent Polyimide–Silica Composite Films Using Polyimide with Carboxylic Acid Groups" Polymers 11, no. 3: 489. https://doi.org/10.3390/polym11030489
APA StyleMoon, K. H., Chae, B., Kim, K. S., Lee, S. W., & Jung, Y. M. (2019). Preparation and Characterization of Transparent Polyimide–Silica Composite Films Using Polyimide with Carboxylic Acid Groups. Polymers, 11(3), 489. https://doi.org/10.3390/polym11030489