Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery
Abstract
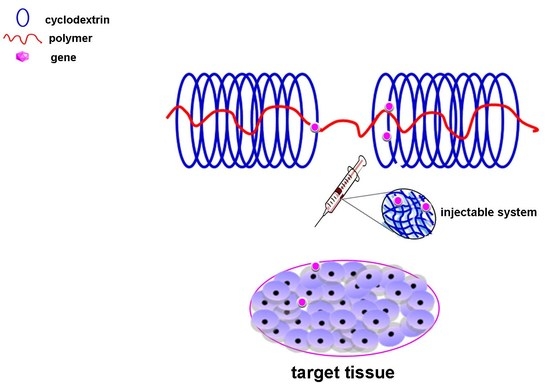
:1. Introduction
1.1. Cyclodextrins
1.2. CD-Based Polypseudorotaxane Hydrogels
2. Gene Transfer Vectors: Basic Concepts
2.1. Nonviral Vectors
2.2. Viral Vectors
3. Controlled Delivery of Gene Transfer Vectors via CD Hydrogels
3.1. Principles of Controlled Gene Delivery
3.2. Controlled Delivery of Gene Transfer Vectors via Supramolecular-Based CD Hydrogels
3.2.1. Controlled Delivery of Nonviral Vectors
3.2.2. Controlled Delivery of Viral Vectors
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Simoes, S.M.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Loh, X.J. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, T.; Higashi, T.; Motoyama, K.; Arima, H. Cyclodextrin-based sustained and controllable release system of insulin utilizing the combination system of self-assembly pegylation and polypseudorotaxane formation. Carbohydr. Polym. 2017, 164, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Harada, A. Supramolecular hydrogels. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–7. [Google Scholar]
- Li, J.; Harada, A.; Kamachi, M. Sol–gel transition during inclusion complex formation between α-cyclodextrin and high molecular weight poly(ethylene glycol)s in aqueous solution. Polym. J. 1994, 26, 1019. [Google Scholar] [CrossRef]
- Harada, A.; Okada, M.; Li, J.; Kamachi, M. Preparation and characterization of inclusion complexes of poly(propylene glycol) with cyclodextrins. Macromolecules 1995, 28, 8406–8411. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, H.B.; Chen, D.H.; Zhang, L.M. Novel supramolecular gelation route to in situ entrapment and sustained delivery of plasmid DNA. J. Colloid Interface Sci. 2011, 364, 566–573. [Google Scholar] [CrossRef]
- Simoes, S.M.; Veiga, F.; Torres-Labandeira, J.J.; Ribeiro, A.C.; Sandez-Macho, M.I.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable pluronic-alpha-cyclodextrin supramolecular gels for sustained delivery of vancomycin. Eur. J. Pharm. Biopharm. 2012, 80, 103–112. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Babicz, H.; Madry, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Cucchiarini, M. Supramolecular polypseudorotaxane gels for controlled delivery of raav vectors in human mesenchymal stem cells for regenerative medicine. Int. J. Pharm. 2017, 531, 492–503. [Google Scholar] [CrossRef]
- Li, J. Supramolecular polymers for potential biomedical applications. Adv. Mater. Res. 2012, 410, 94–97. [Google Scholar] [CrossRef]
- Li, Z.; Yin, H.; Zhang, Z.; Liu, K.L.; Li, J. Supramolecular anchoring of DNA polyplexes in cyclodextrin-based polypseudorotaxane hydrogels for sustained gene delivery. Biomacromolecules 2012, 13, 3162–3172. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Heidari, Z.; Tabassi, S.A.; Tafaghodi, M.; Alibolandi, M.; Tekie, F.S.; Khameneh, B.; Hadizadeh, F. Injectable supramolecular hydrogel from insulin-loaded triblock pcl-peg-pcl copolymer and gamma-cyclodextrin with sustained-release property. AAPS PharmSciTech 2015, 16, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Chua, M.X.; Li, Z.; Loh, X.J.; Wu, Y.L. Injectable supramolecular hydrogels as delivery agents of bcl-2 conversion gene for the effective shrinkage of therapeutic resistance tumors. Adv. Healthc. Mater. 2017, 6, 1700159. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhao, F.; Li, J. Supramolecular polymers based on cyclodextrins for drug and gene delivery. Adv. Biochem. Eng. Biotechnol. 2011, 125, 207–249. [Google Scholar] [PubMed]
- Chen, X.; Qiu, Y.K.; Owh, C.; Loh, X.J.; Wu, Y.L. Supramolecular cyclodextrin nanocarriers for chemo- and gene therapy towards the effective treatment of drug resistant cancers. Nanoscale 2016, 8, 18876–18881. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- De Laporte, L.; Cruz Rea, J.; Shea, L.D. Design of modular non-viral gene therapy vectors. Biomaterials 2006, 27, 947–954. [Google Scholar] [CrossRef]
- Foldvari, M.; Chen, D.W.; Nafissi, N.; Calderon, D.; Narsineni, L.; Rafiee, A. Non-viral gene therapy: Gains and challenges of non-invasive administration methods. J. Control. Release 2016, 240, 165–190. [Google Scholar] [CrossRef]
- Oligino, T.; Ghivizzani, S.; Wolfe, D.; Lechman, E.; Krisky, D.; Mi, Z.; Evans, C.; Robbins, P.; Glorioso, J. Intra-articular delivery of a herpes simplex virus il-1ra gene vector reduces inflammation in a rabbit model of arthritis. Gene Ther. 1999, 6, 1713–1720. [Google Scholar] [CrossRef]
- Marconi, P.; Fraefel, C.; Epstein, A.L. Herpes simplex virus type 1 (HSV-1)-derived recombinant vectors for gene transfer and gene therapy. Methods Mol. Biol. 2015, 1254, 269–293. [Google Scholar] [PubMed]
- Ghivizzani, S.C.; Lechman, E.R.; Kang, R.; Tio, C.; Kolls, J.; Evans, C.H.; Robbins, P.D. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc. Natl. Acad. Sci. USA 1998, 95, 4613–4618. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nagasato, M.; Yoshida, T.; Aoki, K. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci. 2017, 108, 831–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asad, A.S.; Moreno Ayala, M.A.; Gottardo, M.F.; Zuccato, C.; Nicola Candia, A.J.; Zanetti, F.A.; Seilicovich, A.; Candolfi, M. Viral gene therapy for breast cancer: Progress and challenges. Expert Opin. Biol. Ther. 2017, 17, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.Y.; VandenDriessche, T.; Chuah, M.K. Gene therapy for cardiovascular disease: Advances in vector development, targeting, and delivery for clinical translation. Cardiovasc. Res. 2015, 108, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Robbins, P.D.; Ghivizzani, S.C.; Herndon, J.H.; Kang, R.; Bahnson, A.B.; Barranger, J.A.; Elders, E.M.; Gay, S.; Tomaino, M.M.; et al. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum. Gene Ther. 1996, 7, 1261–1280. [Google Scholar] [CrossRef] [PubMed]
- Pagnotto, M.R.; Wang, Z.; Karpie, J.C.; Ferretti, M.; Xiao, X.; Chu, C.R. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007, 14, 804–813. [Google Scholar] [CrossRef]
- Madry, H.; Kaul, G.; Zurakowski, D.; Vunjak-Novakovic, G.; Cucchiarini, M. Cartilage constructs engineered from chondrocytes overexpressing igf-i improve the repair of osteochondral defects in a rabbit model. Eur. Cells Mater. 2013, 25, 229–247. [Google Scholar] [CrossRef]
- Hardcastle, N.; Boulis, N.M.; Federici, T. Aav gene delivery to the spinal cord: Serotypes, methods, candidate diseases, and clinical trials. Expert Opin. Biol. Ther. 2018, 18, 293–307. [Google Scholar] [CrossRef]
- Lubberts, E.; Joosten, L.A.; Chabaud, M.; van Den Bersselaar, L.; Oppers, B.; Coenen-De Roo, C.J.; Richards, C.D.; Miossec, P.; van Den Berg, W.B. IL-4 gene therapy for collagen arthritis suppresses synovial il-17 and osteoprotegerin ligand and prevents bone erosion. J. Clin. Investig. 2000, 105, 1697–1710. [Google Scholar] [CrossRef]
- Calcedo, R.; Wilson, J.M. Humoral immune response to AAV. Front. Immunol. 2013, 4, 341. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Cucchiarini, M. Controlled release strategies for raav-mediated gene delivery. Acta Biomater. 2016, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mallapragada, S.K. Synthetic sustained gene delivery systems. Curr. Top. Med. Chem. 2008, 8, 311–330. [Google Scholar] [PubMed]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, J.; Smiley, E.; Patil, P.; Goldstein, S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999, 5, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Yang, Y.; Hu, Q.; Guo, Z.; Liu, T.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. Injectable supramolecular hydrogel formed from alpha-cyclodextrin and pegylated arginine-functionalized poly(l-lysine) dendron for sustained mmp-9 shrna plasmid delivery. Acta Biomater. 2017, 49, 456–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, Y.-L.; Li, Z.; Loh, X.J. Cyclodextrin-based sustained gene release systems: A supramolecular solution towards clinical applications. Mater. Chem. Front. 2019, 3, 181–192. [Google Scholar] [CrossRef]
- Hu, X.; Wang, N.; Liu, L.; Liu, W. Cyclodextrin-cross-linked diaminotriazine-based hydrogen bonding strengthened hydrogels for drug and reverse gene delivery. J. Biomater. Sci. Polym. Ed. 2013, 24, 1869–1882. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Loh, X.J.; Chen, K.; Li, Z.; Wu, Y.L. Targeted and sustained corelease of chemotherapeutics and gene by injectable supramolecular hydrogel for drug-resistant cancer therapy. Macromol. Rapid Commun. 2019, 40, e1800117. [Google Scholar] [CrossRef]
- Strappe, P.M.; Hampton, D.W.; Cachon-Gonzalez, B.; Fawcett, J.W.; Lever, A. Delivery of a lentiviral vector in a pluronic F127 gel to cells of the central nervous system. Eur. J. Pharm. Biopharm. 2005, 61, 126–133. [Google Scholar] [CrossRef]
- Wang, C.; Pham, P.T. Polymers for viral gene delivery. Expert Opin. Drug Deliv. 2008, 5, 385–401. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. Peo-ppo-peo micelles as effective raav-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–52. [Google Scholar] [CrossRef]
- Cottard, V.; Valvason, C.; Falgarone, G.; Lutomski, D.; Boissier, M.C.; Bessis, N. Immune response against gene therapy vectors: Influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J. Clin. Immunol. 2004, 24, 162–169. [Google Scholar] [CrossRef]


| Polymers/CDs | Vectors | Outcomes | Approaches | Targets | References |
|---|---|---|---|---|---|
| PF68-PLL/α-CD | pDNA-GFP | Sustained pDNA delivery for 80 h; transfection efficiency ~14% | mouse fibroblast cells 3T3 | n.s. | [9] |
| MPEG-PCL-PDMAEMA/α-CD | pDNA-luc | Sustained release of pDNA up to 6 days; transfection efficiency comparable to freshly prepared PEI polyplexes | COS-7 cells | n.s. | [13] |
| PEG-α-CD-cross-linked PVDT | pDNA-luc | Efficient reverse gene transfection of cells cultured on the gel surface | COS-7 cells | n.s. | [39] |
| MPEG-PCL-PEI/α-CD MPEG-PCL-PEIFA/α-CD | pDNA-GFP pDNA-Nur77 | Sustained release of pDNA for 7 days; transfection efficiency of 63% at optimal weight ratio of 1.5; significant inhibition of therapeutic resistant tumor growth with high expression of Bcl-2 proteins Higher efficiency when combining the chemotherapeutic agent paclitaxel and the targeting ability of FA | HEK293 cells, tumor model (BALB/c nude mice) | tumor | [15,40] |
| MPEG-PLLD-Arg/α-CD | pMMP-9 | Controlled release for 6 days; transfection efficiency up to 72%; sustained tumor growth inhibition after 21 days with good biocompatibility | HNE-1 cells, nude mice bearing HNE-1 tumors | tumor | [37] |
| Polymers/CDs | Vectors | Outcomes | Approaches | Targets | References |
|---|---|---|---|---|---|
| CS (or HA)/PF68/α-CD CS (or HA)/T908/α-CD | rAAV-lacZ | Sustained release for 21 days; CS (or HA)/PF68/α-CD gels resulted in the highest rAAV concentrations and sustained levels of transgene expression over time | hMSCs | cartilage repair | [11] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey-Rico, A.; Cucchiarini, M. Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery. Polymers 2019, 11, 514. https://doi.org/10.3390/polym11030514
Rey-Rico A, Cucchiarini M. Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery. Polymers. 2019; 11(3):514. https://doi.org/10.3390/polym11030514
Chicago/Turabian StyleRey-Rico, Ana, and Magali Cucchiarini. 2019. "Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery" Polymers 11, no. 3: 514. https://doi.org/10.3390/polym11030514
APA StyleRey-Rico, A., & Cucchiarini, M. (2019). Supramolecular Cyclodextrin-Based Hydrogels for Controlled Gene Delivery. Polymers, 11(3), 514. https://doi.org/10.3390/polym11030514