Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
2.2. Preparation and Characterization of Soluble Cyclodextrin Crosslinking Polymer
2.3. Preparation and Characterization of Molecular Assembly
2.4. Electrochemical Measurements
2.5. Surface Analyses
2.6. Theoretical Calculations
3. Results and Discussions
3.1. Characterization of Soluble Cyclodextrin Polymer
3.2. Characterization of Molecular Assembly
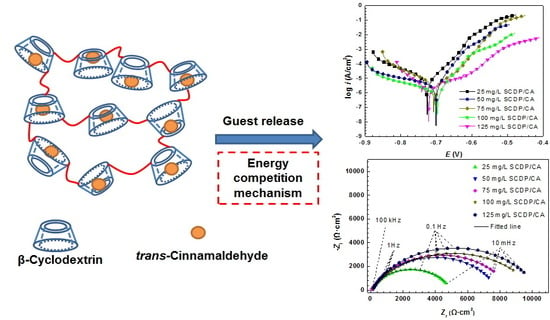
3.3. Corrosion Inhibition Effect of Molecular Assembly
3.4. Surface Analyses
3.5. Theoretical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.M.; Jeong, D.; Cho, E.; Yu, J.H.; Tahir, M.N.; Jung, S. Pentynyl ether of beta-cyclodextrin polymer and silica micro-particles: A new hybrid material for adsorption of phenanthrene from water. Polymers 2017, 9, 10. [Google Scholar] [CrossRef]
- D’Souza, V.T.; Lipkowitz, K.B. Cyclodextrins: Introduction. Chem. Rev. 1998, 98, 1741–1742. [Google Scholar] [CrossRef]
- Asztemborska, M.; Ceborska, M.; Pietrzak, M. Complexation of tropane alkaloids by cyclodextrins. Carbohyd. Polym. 2019, 209, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Saokham, P.; Sá Couto, A.R. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Tang, P.X.; Sun, Q.M.; Suo, Z.L.; Zhao, L.D.; Yang, H.Q.; Xiong, X.N.; Pu, H.Y.; Gan, N.; Li, H. Rapid and efficient removal of estrogenic pollutants from water by using beta- and gamma-cyclodextrin polymers. Chem. Eng. J. 2018, 344, 514–523. [Google Scholar] [CrossRef]
- Xiao, P.; Weibel, N.; Dudal, Y.; Corvini, P.F.X.; Shahgaldian, P. A cyclodextrin-based polymer for sensing diclofenac in water. J. Hazard. Mater. 2015, 299, 412–416. [Google Scholar] [CrossRef]
- Mizuno, S.; Asoh, T.-A.; Takashima, Y.; Harada, A.; Uyama, H. Cyclodextrin cross-linked polymer monolith for efficient removal of environmental pollutants by flow-through method. Polym. Degrad. Stabil. 2019, 160, 136–141. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Obot, I.B.; Sorour, A.A.; Gerengi, H. Exploration of dextran for application as corrosion inhibitor for steel in strong acid environment: Effect of molecular weight, modification, and temperature on efficiency. ACS Appl. Mater. Interfaces 2018, 10, 28112–28129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.K.; Free, M.L.; Woollam, R.; Durnie, W. A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 2017, 90, 159–223. [Google Scholar] [CrossRef]
- Qiang, Y.J.; Zhang, S.T.; Tan, B.C.; Chen, S.J. Evaluation of ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in hcl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Kumar, D.; Jain, V.; Rai, B. Unravelling the mechanisms of corrosion inhibition of iron by henna extract: A density functional theory study. Corros. Sci. 2018, 142, 102–109. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Figa, V.; Privitera, A.; Bruno, M.; Napolitano, A.; Piacente, S. Inhibition of Cor-Ten steel corrosion by “green” extracts of Brassica campestris. Corros. Sci. 2018, 136, 91–105. [Google Scholar] [CrossRef]
- Kim, J.H. Extraction time and temperature affect the extraction efficiencies of coumarin and phenylpropanoids from Cinnamomum cassia bark using a microwave-assisted extraction method. J. Chromatogr. B 2017, 1063, 196–203. [Google Scholar] [CrossRef]
- Cabello, G.; Funkhouser, G.P.; Cassidy, J.; Kiser, C.E.; Lane, J.; Cuesta, A. CO and trans-cinnamaldehyde as corrosion inhibitors of I825, L80-13Cr and N80 alloys in concentrated HCl solutions at high pressure and temperature. Electrochim. Acta 2013, 97, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Avdeev, Y.G.; Kuznetsov, Y.I.; Buryak, A.K. Inhibition of steel corrosion by unsaturated aldehydes in solutions of mineral acids. Corros. Sci. 2013, 69, 50–60. [Google Scholar] [CrossRef]
- Cheng, H.B.; Zhang, Y.M.; Liu, Y.; Yoon, J. Turn-on supramolecular host-guest nanosystems as theranostics for cancer. Chem 2019, 5, 553–574. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, J.P.; Liu, B.C.; Yang, J.K.; Hou, H.J. Recent advances in metalloporphyrins for environmental and energy applications. Chemosphere 2019, 219, 617–635. [Google Scholar] [CrossRef]
- Zhu, J.D.; Zhu, P.; Yan, C.Y.; Dong, X.; Zhang, X.W. Recent progress in polymer materials for advanced lithium-sulfur batteries. Prog. Polym. Sci. 2019, 90, 118–163. [Google Scholar] [CrossRef]
- Kubo, Y.; Nishiyabu, R. White-light emissive materials based on dynamic polymerization in supramolecular chemistry. Polymer 2017, 128, 257–275. [Google Scholar] [CrossRef]
- Fan, B.M.; Hao, H.; Yang, B.; Li, Y. Insights into the inhibition mechanism of a novel supramolecular complex towards the corrosion of mild steel in the condensate water: Experimental and theoretical studies. Res. Chem. Intermed. 2018, 44, 5711–5736. [Google Scholar] [CrossRef]
- Fan, B.M.; Wei, G.; Zhang, Z.; Qiao, N. Characterization of a supramolecular complex based on octadecylamine and β-cyclodextrin and its corrosion inhibition properties in condensate water. Corros. Sci. 2014. [Google Scholar] [CrossRef]
- Fan, B.M.; Wei, G.; Zhang, Z.; Qiao, N. Preparation of supramolecular corrosion inhibitor based on hydroxypropyl-β-cyclodextrin/octadecylamine and its anti-corrosion properties in the simulated condensate water. Anti-Corros. Methods Mater. 2014, 61, 104–111. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instr. 1965, 4, 117–212. [Google Scholar]
- Guo, L.; Kaya, S.; Obot, I.B.; Zheng, X.W.; Qiang, Y.J. Toward understanding the anticorrosive mechanism of some thiourea derivatives for carbon steel corrosion: A combined DFT and molecular dynamics investigation. J. Colloid Interface Sci. 2017, 506, 478–485. [Google Scholar] [CrossRef]
- Benyacoub, A.; Skender, A.; Boutemak, K.; Hadj-Ziane-Zafour, A. Inclusion complexes of Melia azedarach L. seed oil/β-cyclodextrin polymer: Preparation and characterization. Chem. Pap. 2019, 73, 525–534. [Google Scholar] [CrossRef]
- Salazar, S.; Guerra, D.; Yutronic, N.; Jara, P. Removal of aromatic chlorinated pesticides from aqueous solution using beta-cyclodextrin polymers decorated with Fe3O4 nanoparticles. Polymers 2018, 10. [Google Scholar] [CrossRef]
- Deng, S.X.; Liu, H.J.; Qi, C.X.; Yang, A.H.; Li, Z.D. Study on preparation and inclusion behavior of inclusion complexes between beta-cyclodextrin derivatives with benzophenone. J. Incl. Phenom. Macro 2018, 90, 321–329. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Szymanowska-Powalowska, D.; Lewandowska, K.; Blaszczak, W.; Goscianska, J.; Pietrzak, R.; Cielecka-Piontek, J. beta-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016, 99, 24–34. [Google Scholar] [CrossRef]
- Yang, Z.J.; Miao, H.C.; Rui, Z.B.; Ji, H.B. Enhanced formaldehyde removal from air using fully biodegradable chitosan grafted β-Cyclodextrin adsorbent with weak chemical interaction. Polymers 2019, 11, 276. [Google Scholar] [CrossRef]
- Lis, M.; García Carmona, Ó.; García Carmona, C.; Maestá Bezerra, F. Inclusion complexes of citronella oil with β-Cyclodextrin for controlled release in biofunctional textiles. Polymers 2018, 10, 1324. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Uporov, I.V.; Kudryashova, E.V. Thermodynamics and molecular insight in guest-host complexes of fluoroquinolones with beta-cyclodextrin derivatives, as revealed by ATR-FTIR spectroscopy and molecular modeling experiments. Anal. Bioanal. Chem. 2017, 409, 6451–6462. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Z.Y.; Xu, X.M. Inclusion complex of astaxanthin with hydroxypropyl-beta-cyclodextrin: UV, FTIR, H-1 NMR and molecular modeling studies. Carbohyd. Polym. 2012, 89, 492–496. [Google Scholar] [CrossRef]
- Ding, X.Z.; Zheng, M.K.; Lu, J.M.; Zhu, X.Y. Preparation and evaluation of binary and ternary inclusion complexes of fenofibrate/hydroxypropyl-beta-cyclodextrin. J. Incl. Phenom. Macro 2018, 91, 17–24. [Google Scholar] [CrossRef]
- Kumar, S.; Goyal, M.; Vashisht, H.; Sharma, V.; Bahadur, I.; Ebenso, E.E. Ionic salt (4-ethoxybenzyl)-triphenylphosphonium bromide as a green corrosion inhibitor on mild steel in acidic medium: Experimental and theoretical evaluation. RSC Adv. 2017, 7, 31907–31920. [Google Scholar] [CrossRef]
- Mishra, A.; Verma, C.; Lgaz, H.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Synthesis, characterization and corrosion inhibition studies of N-phenyl-benzamides on the acidic corrosion of mild steel: Experimental and computational studies. J. Mol. Liq. 2018, 251, 317–332. [Google Scholar] [CrossRef]
- Sukul, D.; Pal, A.; Saha, S.K.; Satpati, S.; Adhikari, U.; Banerjee, P. Newly synthesized quercetin derivatives as corrosion inhibitors for mild steel in 1 M HCl: Combined experimental and theoretical investigation. Phys. Chem. Chem. Phys. 2018, 20, 6562–6574. [Google Scholar] [CrossRef]
- Tang, J.L.; Hu, Y.X.; Han, Z.Z.; Wang, H.; Zhu, Y.Q.; Wang, Y.; Nie, Z.; Wang, Y.Y. Experimental and theoretical study on the synergistic inhibition effect of pyridine derivatives and sulfur-containing compounds on the corrosion of carbon steel in CO2-saturated 3.5 wt.% NaCl solution. Molecules 2018, 23, 3270. [Google Scholar] [CrossRef]
- Dagdag, O.; El Harfi, A.; Cherkaoui, O.; Safi, Z.; Wazzan, N.; Guo, L.; Akpan, E.D.; Verma, C.; Ebenso, E.E.; Jalgham, R.T.T. Rheological, electrochemical, surface, DFT and molecular dynamics simulation studies on the anticorrosive properties of new epoxy monomer compound for steel in 1 M HCl solution. RSC Adv. 2019, 9, 4454–4462. [Google Scholar] [CrossRef]
- Jamil, D.M.; Al-Okbi, A.K.; Al-Baghdadi, S.B.; Al-Amiery, A.A.; Kadhim, A.; Gaaz, T.S.; Kadhum, A.A.H.; Mohamad, A.B. Experimental and theoretical studies of Schiff bases as corrosion inhibitors. Chem. Cent. J. 2018, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Hughes, A.E.; Deacon, G.B.; Junk, P.C.; Hinton, B.R.W.; Forsyth, M.; Mardel, J.I.; Somers, A.E. A study of rare-earth 3-(4-methylbenzoyl)-propanoate compounds as corrosion inhibitors for AS1020 mild steel in NaCl solutions. Corros. Sci. 2018, 145, 199–211. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, S.H.; Zhang, S.T. Experimental and theoretical studies of benzalkonium chloride as an inhibitor for carbon steel corrosion in sulfuric acid. J. Ind. Eng. Chem. 2015, 24, 174–180. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lgaz, H.; Lin, Y. Synthesis and investigation of pyran derivatives as acidizing corrosion inhibitors for N80 steel in hydrochloric acid: Theoretical and experimental approaches. J. Alloys Compd. 2018, 762, 347–362. [Google Scholar] [CrossRef]
- Messali, M.; Larouj, M.; Lgaz, H.; Rezki, N.; Al-Blewi, F.F.; Aouad, M.R.; Chaouiki, A.; Salghi, R.; Chung, I.-M. A new schiff base derivative as an effective corrosion inhibitor for mild steel in acidic media: Experimental and computer simulations studies. J. Mol. Struct. 2018, 1168, 39–48. [Google Scholar] [CrossRef]
- Cen, H.Y.; Cao, J.J.; Chen, Z.Y.; Guo, X.P. 2-Mercaptobenzothiazole as a corrosion inhibitor for carbon steel in supercritical CO2-H2O condition. Appl. Surf. Sci. 2019, 476, 422–434. [Google Scholar] [CrossRef]
- Chen, J.X.; Wang, C.; Han, J.; Hu, B.S.; Wang, C.B.; Zhong, Y.L.; Xu, H. Corrosion inhibition performance of threonine-modified polyaspartic acid for carbon steel in simulated cooling water. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Verma, C.; Lgaz, H.; Verma, D.K.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. Molecular dynamics and Monte Carlo simulations as powerful tools for study of interfacial adsorption behavior of corrosion inhibitors in aqueous phase: A review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
- Wang, T.Y.; Zou, C.J.; Li, D.X.; Chen, Z.L.; Liu, Y.; Li, X.K.; Li, M. Theoretical investigation on cyclodextrin inclusion complexes with organic phosphoric acid as corrosion inhibitor. Acta Phys.-Chim. Sin. 2015, 31, 2294–2302. [Google Scholar] [CrossRef]
- Liao, L.L.; Mo, S.; Luo, H.Q.; Li, N.B. Corrosion protection for mild steel by extract from the waste of lychee fruit in HCl solution: Experimental and theoretical studies. J. Colloid Interface Sci. 2018, 520, 41–49. [Google Scholar] [CrossRef]
- Abdulazeez, I.; Zeino, A.; Kee, C.W.; Al-Saadi, A.A.; Khaled, M.; Wong, M.W.; Al-Sunaidi, A.A. Mechanistic studies of the influence of halogen substituents on the corrosion inhibitive efficiency of selected imidazole molecules: A synergistic computational and experimental approach. Appl. Surf. Sci. 2019, 471, 494–505. [Google Scholar] [CrossRef]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]













| Concentration | Ecorr (V) | icorr (×10−1 mA/cm2) | ba (mV/dec) | bc (mV/dec) | IE1 (%) |
|---|---|---|---|---|---|
| Blank | −0.742 | 3.93 | 56.5 | −85.8 | — |
| 125 mg/L SCDP | −0.728 | 2.18 | 98.7 | −83.6 | 44.5 |
| 25 mg/L SCDP/CA | −0.726 | 0.761 | 83.4 | −98.9 | 80.6 |
| 50 mg/L SCDP/CA | −0.701 | 0.468 | 106.9 | −81.2 | 88.1 |
| 75 mg/L SCDP/CA | −0.706 | 0.313 | 164.4 | −89.2 | 92.0 |
| 100 mg/L SCDP/CA | −0.698 | 0.311 | 171.3 | −87.1 | 92.1 |
| 125 mg/L SCDP/CA | −0.721 | 0.307 | 186.6 | −106.4 | 92.2 |
| Concentration | Rs (Ω·cm2) | Cdl (μF/cm2) | n | Rp (Ω·cm2) | IE2 (%) | Rpl (Ω·cm2) |
|---|---|---|---|---|---|---|
| Blank | 11.5 | 782.3 | 0.89 | 1006.2 ±12.9 | — | 1018.7 |
| 125 mg/L SCDP | 21.7 | 511.2 | 0.91 | 1859.9 ± 37.7 | 45.9 | 1798.9 |
| 25 mg/L SCDP/CA | 28.3 | 348.8 | 0.93 | 4814.3 ± 61.1 | 79.1 | 4827.3 |
| 50 mg/L SCDP/CA | 33.6 | 226.4 | 0.89 | 7565.4 ± 50.9 | 86.7 | 7701.5 |
| 75 mg/L SCDP/CA | 50.1 | 258.9 | 0.87 | 8385.2 ± 94.4 | 88.0 | 8381.4 |
| 100 mg/L SCDP/CA | 30.9 | 185.1 | 0.89 | 9891.6 ± 68.3 | 89.8 | 9780.2 |
| 125 mg/L SCDP/CA | 55.8 | 180.7 | 0.93 | 10565.5 ± 72.7 | 90.5 | 10011.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Fan, B.; Zhou, T.; Hao, H.; Yang, B.; Sun, H. Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies. Polymers 2019, 11, 635. https://doi.org/10.3390/polym11040635
Ma Y, Fan B, Zhou T, Hao H, Yang B, Sun H. Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies. Polymers. 2019; 11(4):635. https://doi.org/10.3390/polym11040635
Chicago/Turabian StyleMa, Yucong, Baomin Fan, Tingting Zhou, Hua Hao, Biao Yang, and Hui Sun. 2019. "Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies" Polymers 11, no. 4: 635. https://doi.org/10.3390/polym11040635
APA StyleMa, Y., Fan, B., Zhou, T., Hao, H., Yang, B., & Sun, H. (2019). Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies. Polymers, 11(4), 635. https://doi.org/10.3390/polym11040635