Poly (2-hydroxyethylmethacrylate –co–methylmethacrylate)/Lignocaine Contact Lens Preparation, Characterization, and in vitro Release Dynamic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PHEMMA
2.3. Synthesis of PHEMMA/LIG and PHEMMAC/LIG
2.4. Characterization
2.4.1. CHN Analysis
2.4.2. H-NMR Analysis
2.4.3. DSC Analysis
2.4.4. Fourier Transform Infrared Analysis
2.4.5. X-ray Diffraction Analysis
2.4.6. Water Content
2.4.7. Mechanical Testing
2.4.8. UV-Visible Analysis
2.4.9. Refractive Indices
2.4.10. Cell Viability
3. Results and Discussion
3.1. Characterization
3.1.1. CHN Analysis
3.1.2. H-NMR Analysis
3.1.3. DSC Analysis
3.1.4. FTIR Analysis
3.1.5. X-ray Diffraction Analysis
3.1.6. Water content at equilibrium, EWC
3.1.7. Mechanical Testing
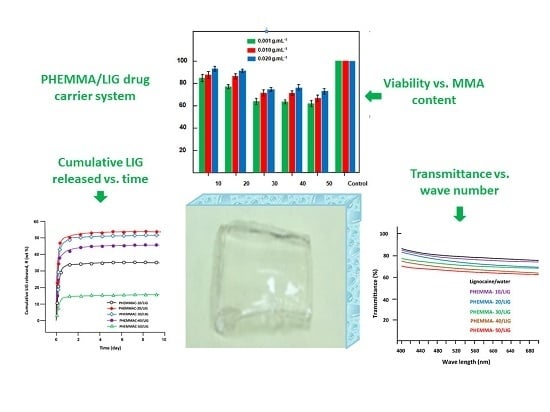
3.1.8. Transparency
3.1.9. Refractive Indices
3.1.10. Cell Viability
3.2. In Vitro LIG Release
3.2.1. Kinetics Release of LIG
3.2.2. Diffusion Behavior of Lignocaine
3.2.3. Effects of the MMA/HEMA Composition
3.2.4. Effects of the water content at equilibrium
3.2.5. Performance of the PHEMMAC/LIG Drug-Carrier System
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nagarsenker, M.S.; Londhe, V.Y.; Nadkarni, G.D. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int. J. Pharm. 1990, 190, 63–71. [Google Scholar] [CrossRef]
- Bourlais, C.L.; Acar, L.; Zia, H.; Sado, P.A.; Needham, T.; Leverge, R. Ophthalmic drug delivery systems–recent advances. Prog. Retin. Eye. Res. 1998, 17, 33–58. [Google Scholar] [CrossRef]
- Lang, J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug. Deliv. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic side effects of eye drops: A pharmacokinetic perspective. Clin. Ophthalmol. 2016, 10, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Mäenpää, J.; Pelkonen, O. Cardiac safety of ophthalmic timolol. Expert. Opin. Drug. Saf. 2016, 15, 1549–1561. [Google Scholar] [CrossRef]
- Hegde, R.; Verma, A.; Ghosh, A. Microemulsion: New Insights into the Ocular Drug Delivery. ISRN Pharmaceutics 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zhang, Y.; Zhang, W.S. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390. [Google Scholar]
- Cereda, C.M.; Brunetto, G.B.; de Araújo, D.R.; de Paula, E. Liposomal formulations of prilocaine, lidocaine and mepivacaine prolong analgesic duration. Can. J. Anesth. 2006, 53, 1092–1097. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Ophthalmic drug delivery through contact lenses. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2342–2347. [Google Scholar] [CrossRef]
- Jung, H.J.; Chauhan, A.A. Ophthalmic drug delivery by contact lenses. Expert Rev. Ophthalmol. 2012, 7, 199–201. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Sriwidodo, S.; Mita, S.R.; Ravi, K. Drug Delivery System by Hydrogel Soft Contact Lens Materials: A Review. J. Pharm. Sci.Res. 2018, 10, 254–256. [Google Scholar]
- Jenkins, F.A.; White, H.E. Fundamentals of Optics; Tata McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Malhotra, S.L.; Minh, L.Y.; Blanchard, L.P. Thermal Decomposition and Glass Transition Temperature of Poly (methy1 Methacrylate) and Poly (isobuty1 Methacrylate). J. Macromol. Sci. Chem. 1983, A19, 579–600. [Google Scholar] [CrossRef]
- Morita, S.; Ye, S.; Li, G.F.; Osawa, M. Effect of glass transition temperature (Tg) on the absorption of bisphenol A in poly(acrylate)s thin films. Vib. Spectrosc. 2004, 35, 15–19. [Google Scholar] [CrossRef]
- Kang, L.; Jun, H.W.; Mani, N. Preparation and characterization of two-phase melt systems of LID. Int. J. Pharmaceut. 2001, 222, 35–44. [Google Scholar] [CrossRef]
- Morita, S. Hydrogen-bonds structure in poly (2-hydroxyethyl methacrylate) studied by temperature-dependent infrared spectroscopy, Frontier in Chemistry. Polym. Chem. 2014, 2, 1–5. [Google Scholar]
- Wei, Y.; Nedley, M.P.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H.S. Masking the Bitter Taste of Injectable Lidocaine HCl Formulation for Dental Procedures. AAPS. Pharm. Sci. Tech. 2015, 16, 455–465. [Google Scholar] [CrossRef]
- Luo, Y.; Dalton, P.D.; Shoichet, M.S. Investigating the Properties of Novel Poly (2-hydroxyethyl methacrylate-co-methyl methacrylate) Hydrogel Hollow Fiber Membranes. Chem. Mater. 2001, 13, 4087–4093. [Google Scholar] [CrossRef]
- Ishiyama, C.; Higo, Y. Effects of Humidity on Young’s Modulus in Poly (methyl methacrylate). J. Polym. Sci. Part B Polym. Phys. 2002, 40, 460–465. [Google Scholar] [CrossRef]
- Bhamra, T.S.; Tighe, B.J. Mechanical properties of contact lenses: The contribution of measurement techniques and clinical feedback to 50 years of materials development. Cont. Lens Anterior Eye 2017, 40, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Varikooty, J.; Keir, N.; Woods, C.A.; Fonn, D. Measurement of the refractive index of soft contact lenses during wear. Eye Contact Lens 2010, 36, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.J. Handbook of Optical Materials; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Vargun, E.; Sankiri, M.; Arani, B.I.; Sankiri, N.D.; Usanmaz, A.I. Synthesis and Characterization of 2-Hydroxyethyl Methacrylate (HEMA) and Methyl Methacrylate (MMA) Copolymer Used as Biomaterial. J. Macromol. Sci. Part A Pure Appl. Chem. 2010, 47, 235–240. [Google Scholar] [CrossRef]
- Lin, M.; Wang, H.; Meng, S.; Zhong, W.; Li, Z.; Cai, R.; Chen, Z.; Zhou, X.; Du, Q. Structure and release behavior of PMMA/silica composite drug delivery system. J. Pharm. Sci. 2007, 96, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, C.S.; Radomsky, M.L.; Salzman, W.M.; Hilton, J.; Brem, H. Polymeric controlled release of dexamethasone in normal rat brain. J. Control. Rel. 1991, 16, 331–340. [Google Scholar] [CrossRef]
- Cypes, S.H.; Saltzman, W.M.; Giammelis, E.P. Organosilicate – Polymer drug delivery systems: Controlled release and enhanced mechanical properties. J. Control. Rel. 2003, 90, 163–169. [Google Scholar] [CrossRef]
- Frank, A.; Rath, S.K.; Venkatraman, S.S. Controlled release from bioerodible polymers: Effect of drug type and polymer composition. J. Control. Rel. 2005, 102, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Dilmi, A.; Bartil, T.; Yahia, N.; Benneghmouche, Z. Hydrogels based on 2-hydroxyethylmethacrylate and chitosan: Preparation, swelling behavior, and drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 502–509. [Google Scholar] [CrossRef]
- Kim, S.K. Small intestine transit time in the normal small bowel study. Am. J. Roentgenol. 1968, 104, 522–524. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Erdogan, A.; Rao, S.S.C. How to assess regional and whole gut transit time with wireless motility capsule. J. Neurogastroenterol. Motil. 2014, 20, 265–270. [Google Scholar] [CrossRef]
- Khalid, S.H.; Qadir, M.I.; Massud, A.; Ali, M.; Rasool, M.H. Effect of degree of cross-linking on swelling and drug release behaviour of poly(methyl methacrylate-co-itaconic acid) [P(MMA/IA)] hydrogels for site specific drug delivery. J. Drug. Deliv. Sci. Technol. 2009, 19, 413–418. [Google Scholar] [CrossRef]
- Wan, L.S.C.; Heng, P.W.S.; Wong, L.F. Relationship Between Swelling and Swelling and Drug Release in a hydrophilic Matrix. Drug. Develop. Ind. Pharm. 1993, 19, 1201–1210. [Google Scholar] [CrossRef]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Dinu-PÎrvu, C.; Ivana, S. A Study of the influence of crosslinking degree on the Physicochemical Properties of Gelatin Microparticles. Cellulose Chem. Technol. 2013, 47, 721–726. [Google Scholar]
- Ferreira, L.; Vidal, M.M.; Gil, M.H. Evaluation of poly (2-hydroxyethyl methacrylate) gels as drug delivery systems at different pH values. Int. J. Pharm. 2000, 194, 169–180. [Google Scholar] [CrossRef]
- Carbinatto, F.M.; de Castro, A.D.; Evangelista, R.C.; Cury, B.S.F. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharmaceut. Sci. 2014, 9, 27–34. [Google Scholar] [CrossRef] [Green Version]
















| System PHEMMA | MMA (g) | HEMA (g) | AIBN (mg) | THF (mL) |
|---|---|---|---|---|
| PHEMMA-10 | 1.0 | 9.0 | 10 | 20.0 |
| PHEMMA-20 | 2.0 | 8.0 | 10 | 20.0 |
| PHEMMA-30 | 3.0 | 7.0 | 10 | 20.0 |
| PHEMMA-40 | 4.0 | 6.0 | 10 | 20.0 |
| PHEMMA-50 | 5.0 | 5.0 | 10 | 20.0 |
| System PHEMMA/LIG | MMA (g) | HEMA (g) | AIBN (mg) | LIG (g) | LIG (wt %) |
|---|---|---|---|---|---|
| PHEMMA-10/LIG | 1.0 | 9.0 | 10 | 0.12 | 1.20 |
| PHEMMA-20/LIG | 2.0 | 8.0 | 10 | 0.12 | 1.20 |
| PHEMMA-30/LIG | 3.0 | 7.0 | 10 | 0.12 | 1.20 |
| PHEMMA-40/LIG | 4.0 | 6.0 | 10 | 0.12 | 1.20 |
| PHEMMA-50/LIG | 5.0 | 5.0 | 10 | 0.12 | 1.20 |
| System | Element (CHN) | HEMA (wt %) | MMA (wt %) | LIG (wt %) | |||
|---|---|---|---|---|---|---|---|
| C (wt %) | H (wt %) | O (wt %) | N (wt %) | 1H-NMR | CHN | ||
| PHEMMAC-10/LIG | 56.23 | 7.73 | 35.80 | 0.23 | 85.67 | 13.21 | 1.05 |
| PHEMMAC-20/LIG | 56.53 | 7.75 | 35.48 | 0.24 | 78.21 | 20.67 | 1.06 |
| PHEMMAC-30/LIG | 56.92 | 7.78 | 35.05 | 0.24 | 68.34 | 30.54 | 1.05 |
| PHEMMAC-40/LIG | 57.41 | 7.81 | 34.52 | 0.25 | 57.12 | 41.76 | 1.07 |
| PHEMMAC-50/LIG | 57.89 | 7.84 | 34.00 | 0.26 | 46.56 | 52.32 | 1.07 |
| Polymer and Copolymer | Tg (°C) | PHEMMA/LIG Composite | Tg (°C) |
|---|---|---|---|
| PHEMA | 83 | - | - |
| PHEMMA-10 | 86 | PHEMMA-10/LIG | 84 |
| PHEMMA-20 | 88 | PHEMMA-20/LIG | 86 |
| PHEMMA-30 | 90 | PHEMMA-30/LIG | 88 |
| PHEMMA-40 | 92 | PHEMMA-40/LIG | 90 |
| PHEMMA-50 | 95 | PHEMMA-50/LIG | 93 |
| PMMA | 105 | - | - |
| System | υ (C=O) (cm−1) | υ (O–H) (cm−1) | System | υ (C=O) (cm−1) | υ (O–H) (cm−1) |
|---|---|---|---|---|---|
| PMMA | 1732 | - | Lignocaine | 1722 | 3670–3125 |
| PHEMMA-10 | 1730 | 3650–3125 | PHEMMA-10/LIG | 1724 | 3672–3125 |
| PHEMMA- 20 | 1728 | 3650–3130 | PHEMMA- 20/LIG | 1724 | 3675–3125 |
| PHEMMA- 30 | 1727 | 3650–3135 | PHEMMA- 30/LIG | 1725 | 3700–3125 |
| PHEMMA- 50 | 1727 | 3690–3145 | PHEMMA-50/LIG | 1726 | 3725–3125 |
| System | EWC (wt %) | Elastic Modulus (kPa) |
|---|---|---|
| PHEMA | 43.2 ± 0.2 | 17 ± 4 |
| PHEMMA-10 | 32.0 ± 0.2 | 123 ± 12 |
| PHEMMA-20 | 21.4 ± 0.3 | 317 ± 11 |
| PHEMMA-30 | 13.7 ± 0.4 | 782 ± 25 |
| PHEMMA-40 | 8.3 ± 0.5 | 1880 ± 25 |
| PHEMMA-50 | 5.5 ± 0.5 | 25,058 ± 32 |
| PMMA | 1.3 ± 0.5 | 32,000 ± 43 |
| Copolymer | Refractive Index at 37 °C | Composite | Refractive Index at 37 °C | ||||
|---|---|---|---|---|---|---|---|
| λ (nm) | 405 | 532 | 670 | λ (nm) | 405 | 532 | 670 |
| PHEMA | 1.5220 | 1.5125 | 1.4817 | - | - | - | - |
| PHEMMAC-10 | 1.5205 | 1.5014 | 1.4760 | PHEMMAC-10/LIG | 1.5256 | 1.5082 | 1.4783 |
| PHEMMAC-20 | 1.5175 | 1.4801 | 1.4712 | PHEMMAC-20/LIG | 1.5200 | 1.4862 | 1.4734 |
| PHEMMAC-30 | 1.5140 | 1.4783 | 1.4567 | PHEMMAC-30/LIG | 1.5183 | 1.4812 | 1.4583 |
| PHEMMAC-40 | 1.5032 | 1.4619 | 1.4421 | PHEMAC-40/LIG | 1.5112 | 1.4657 | 1.4465 |
| PHEMMAC-50 | 1.4913 | 1.4504 | 1.4326 | PHEMMAC-50/LIG | 1.5002 | 1.4552 | 1.4382 |
| PMMA | 1.4878 | 1.4434 | 1.4333 | - | - | - | - |
| Drug-Carrier System | Stable Zone (h) | LIG Released (wt %) | Release Rate (wt %.h−1) |
|---|---|---|---|
| PHEMMAC-10/LIG | 0–5 5–168 | 33.50 ± 0.15 0.25 ± 0.08 | 6.50 ± 0.03 0.002 ± 0.001 |
| PHEMMAC-20/LIG | 0–5 18–168 | 46.00 ± 0.25 4.80 ± 0.32 | 9.40 ± 0.05 0.03 ± 0.01 |
| PHEMMAC-30/LIG | 0–5 36–168 | 47.00 ± 0.25 5.00 ± 0.32 | 9.40 ± 0.05 0.04 ± 0.01 |
| PHEMMAC-40/LIG | 0–5 120–168 | 7.80 ± 0.15 1.61 ± 0.38 | 1.96 ± 0.03 0.03 ± 0.01 |
| PHEMMAC-50/LIG | 0–5 5–168 | 5.03 ± 0.20 2.01 ± 0.63 | 1.00 ± 0.04 0.01 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouak, T.; Saeed, W.S.; Al-Hafi, N.M.; Al-Odayni, A.-B.; Alghamdi, A.A.; Bedja, I. Poly (2-hydroxyethylmethacrylate –co–methylmethacrylate)/Lignocaine Contact Lens Preparation, Characterization, and in vitro Release Dynamic. Polymers 2019, 11, 917. https://doi.org/10.3390/polym11050917
Aouak T, Saeed WS, Al-Hafi NM, Al-Odayni A-B, Alghamdi AA, Bedja I. Poly (2-hydroxyethylmethacrylate –co–methylmethacrylate)/Lignocaine Contact Lens Preparation, Characterization, and in vitro Release Dynamic. Polymers. 2019; 11(5):917. https://doi.org/10.3390/polym11050917
Chicago/Turabian StyleAouak, Taieb, Wassem Sharaf Saeed, Nawaf M. Al-Hafi, Abdel-Basit Al-Odayni, Abdulaziz Ali Alghamdi, and Idriss Bedja. 2019. "Poly (2-hydroxyethylmethacrylate –co–methylmethacrylate)/Lignocaine Contact Lens Preparation, Characterization, and in vitro Release Dynamic" Polymers 11, no. 5: 917. https://doi.org/10.3390/polym11050917
APA StyleAouak, T., Saeed, W. S., Al-Hafi, N. M., Al-Odayni, A.-B., Alghamdi, A. A., & Bedja, I. (2019). Poly (2-hydroxyethylmethacrylate –co–methylmethacrylate)/Lignocaine Contact Lens Preparation, Characterization, and in vitro Release Dynamic. Polymers, 11(5), 917. https://doi.org/10.3390/polym11050917