Structure and Mechanical Properties of Multi-Walled Carbon Nanotubes-Filled Isotactic Polypropylene Composites Treated by Pressurization at Different Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of iPP/MWCNTs Composites
2.3. Tensile Experiments
2.4. Characterizations
2.4.1. Wide-Angle and Small-Angle X-ray Measurements
2.4.2. Differential Scanning Calorimeter
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. Atomic Force Microscope
3. Results and Discussion
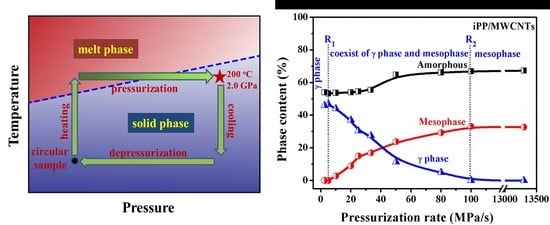
3.1. Pressurization-Induced Formation of Meso-iPP/MWCNTs Composites
3.2. Effect of Pressurization Rate on Polymorphism of Crystallization
3.3. Effect of Pressurization Rate on Nanoscale Structure of the Composites
3.4. Thermal Preformance of the iPP/MWCNTs Nanocomposites
3.5. Mechanical Properties of the iPP/MWCNTs Nanocomposites
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Okada, K.; Hikima, Y.; Ohshima, M.; Sekiguchi, T.; Yano, H. Effect of Cellulose Nanofiber (CNF) Surface Treatment on Cellular Structures and Mechanical Properties of Polypropylene/CNF Nanocomposite Foams via Core-Back Foam Injection Molding. Polymers 2019, 11, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bu, X.; Feng, Q.; Li, D. Cellulose Nanofiber/Carbon Nanotube Conductive Nano-Network as a Reinforcement Template for Polydimethylsiloxane Nanocomposite. Polymers 2018, 10, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Bhuiyan, M.K.H.; Rahman, M.M.; Mina, M.F.; Islam, M.R.; Gafur, M.A.; Begum, A. Crystalline morphology and properties of multi-walled carbon nanotube filled isotactic polypropylene nanocomposites: Influence of filler size and loading. Compos. Part A 2013, 52, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Manchado, M.A.L.; Valentini, L.; Biagiotti, J.; Kenny, J.M. Thermal and mechanical properties of single-walled carbon nanotubes–polypropylene composites prepared by melt processing. Carbon 2005, 43, 1499–1505. [Google Scholar] [CrossRef]
- Logakis, E.; Pollatos, E.; Pandis, C.; Peoglos, V.; Zuburtikudis, I.; Delides, C.G.; Vatalis, A.; Gjoka, M.; Syskakis, E.; Viras, K.; et al. Structure–property relationships in isotactic polypropylene/multi-walled carbon nanotubes nanocomposites. Compos. Sci. Technol. 2010, 70, 328–335. [Google Scholar] [CrossRef]
- Zhao, K.; Li, S.; Huang, M.; Shi, X.; Zheng, G.; Liu, C.; Dai, K.; Shen, C.; Yin, R.; Guo, J.Z. Remarkably anisotropic conductive MWCNTs/polypropylene nanocomposites with alternating microlayers. Chem. Eng. J. 2019, 358, 924–935. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Q.; Li, K.; Hu, G.-H. Preparation of polypropylene/carbon nanotube composite powder with a solid-state mechanochemical pulverization process. J. Appl. Polym. Sci. 2004, 93, 378–386. [Google Scholar] [CrossRef]
- Coleman, J.N.; Cadek, M.; Blake, R.; Nicolosi, V.; Ryan, K.P.; Belton, C.; Fonseca, A.; Nagy, J.B.; Gun’ko, Y.K.; Blau, W.J. High Performance Nanotube-Reinforced Plastics: Understanding the Mechanism of Strength Increase. Adv. Funct. Mater. 2004, 14, 791–798. [Google Scholar] [CrossRef]
- Chang, B.; Schneider, K.; Heinrich, G.; Li, Y.; Zheng, G.; Häußler, L.; Liu, C.; Shen, C. Competition effect of shear-induced nuclei and multiwalled carbon nanotubes (MWCNT) on β-isotactic polypropylene (iPP) formation in preshear injection-molded iPP/MWCNT nanocomposites. Polym. Compos. 2018, 39, 1149–1158. [Google Scholar] [CrossRef]
- Stan, F.; Sandu, L.I.; Fetecau, C. Effect of processing parameters and strain rate on mechanical properties of carbon nanotube–filled polypropylene nanocomposites. Compos. Part B 2014, 59, 109–122. [Google Scholar] [CrossRef]
- Grady, B.P.; Pompeo, F.; Shambaugh, R.L.; Resasco, D.E. Nucleation of Polypropylene Crystallization by Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2002, 106, 5852–5858. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Z. Role of multi-wall carbon nanotube network in composites to crystallization of isotactic polypropylene matrix. Polymer 2008, 49, 330–338. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, W.; Zhu, L.; Wong, C.-P.; Bucknall, D.G. γ-Form Transcrystals of Poly(propylene) Induced by Individual Carbon Nanotubes. Macromol. Chem. Phys. 2010, 211, 1348–1354. [Google Scholar] [CrossRef]
- Ning, N.; Wei, Z.; Yan, J.; Fan, X.; Tang, C.; Qiang, F. Effect of surface “groove” structure of carbon nanotube bundles on the formation of nanohybrid shish kebab. J. Mater. Res. 2012, 27, 2812–2818. [Google Scholar] [CrossRef]
- Coburn, N.; Douglas, P.; Kaya, D.; Gupta, J.; McNally, T. Isothermal and non-isothermal crystallization kinetics of composites of poly(propylene) and MWCNTs. Adv. Ind. Eng. Polym. Res. 2018, 1, 99–110. [Google Scholar] [CrossRef]
- Banerjee, J.; Parija, S.; Panwar, A.S.; Mukhopadhyay, K.; Saxena, A.K.; Bhattacharyya, A.R. Isothermal crystallization kinetics of polypropylene in melt-mixed composites of polypropylene and multi-walled carbon nanotubes. Polym. Eng. Sci. 2017, 57, 1136–1146. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Yuan, C.; Jia, R.; Shao, C.; Wang, M.; Hong, S. A Study of the Pressure-Induced Solidification of Polymers. Polymers 2018, 10, 847–856. [Google Scholar] [CrossRef]
- Jia, R.; Shao, C.G.; Su, L.; Huang, D.H.; Liu, X.R.; Hong, S.M. Rapid compression induced solidification of bulk amorphous sulfur. J. Phys. D Appl. Phys. 2007, 40, 3763–3766. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, R.; Shao, C.; Wang, Y.; Shen, C. Cold crystallization behavior of glassy poly(lactic acid) prepared by rapid compression. Polym. Eng. Sci. 2015, 55, 359–366. [Google Scholar] [CrossRef]
- Huang, J.; Fu, X.; Shao, C.; Ma, Z.; Wang, Y.; Liu, C.; Shen, C. High-pressure induced formation of isotactic polypropylene mesophase: Synergistic effect of pressure and pressurization rate. Polym. Eng. Sci. 2019, 59, 439–446. [Google Scholar] [CrossRef]
- Fu, X.; Jia, W.; Li, X.; Wang, Y.; Wang, Z.; Liu, C.; Shen, C.; Shao, C. Phase transitions of the rapid-compression-induced mesomorphic isotactic polypropylene under high-pressure annealing. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 651–661. [Google Scholar] [CrossRef]
- Angelloz, C.; Fulchiron, R.; Douillard, A.; Chabert, B. Crystallization of Isotactic Polypropylene under High Pressure (γ Phase). Macromolecules 2000, 33, 4138–4145. [Google Scholar] [CrossRef]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene. II: The morphology of the γ-form crystallized at 200 M Pa. Polymer 1997, 38, 5725–5733. [Google Scholar] [CrossRef]
- Lezak, E.; Bartczak, Z. Plastic Deformation of the γ Phase Isotactic Polypropylene in Plane-Strain Compression at Elevated Temperatures. Macromolecules 2007, 40, 4933–4941. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Yang, S.; Ren, P.; Zhang, Q.; Li, Z. Polypropylene films with high barrier performance via crystal morphology manipulation. J. Mater. Sci. 2017, 52, 5449–5461. [Google Scholar] [CrossRef]
- Li, X.; Su, F.; Ji, Y.; Tian, N.; Lu, J.; Wang, Z.; Qi, Z.; Li, L. Influence of the memory effect of a mesomorphic isotactic polypropylene melt on crystallization behavior. Soft Matter 2013, 9, 8579–8588. [Google Scholar] [CrossRef]
- Hong, S.M.; Chen, L.Y.; Liu, X.R.; Wu, X.H.; Su, L. High pressure jump apparatus for measuring Grűneisen parameter of NaCl and studying metastable amorphous phase of poly (ethylene terephthalate). Rev. Sci. Instrum. 2005, 76, 053905–053910. [Google Scholar] [CrossRef]
- Kang, J.; He, J.; Chen, Z.; Yu, H.; Chen, J.; Yang, F.; Cao, Y.; Xiang, M. Investigation on the crystallization behavior and polymorphic composition of isotactic polypropylene/multi-walled carbon nanotube composites nucleated with β-nucleating agent. J. Therm. Anal. Calorim. 2014, 119, 1769–1780. [Google Scholar] [CrossRef]
- Van Drongelen, M.; Van Erp, T.B.; Peters, G.W.M. Quantification of non-isothermal, multi-phase crystallization of isotactic polypropylene: The influence of cooling rate and pressure. Polymer 2012, 53, 4758–4769. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Di Girolamo, R.; de Ballesteros, O.R. Crystallization of the mesomorphic form and control of the molecular structure for tailoring the mechanical properties of isotactic polypropylene. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 677–699. [Google Scholar] [CrossRef]
- Fisher, F.T.; Bradshaw, R.D.; Brinson, L.C. Effects of nanotube waviness on the modulus of nanotube-reinforced polymers. Appl. Phys. Lett. 2002, 80, 4647–4649. [Google Scholar] [CrossRef] [Green Version]
- Murthy, N.S.; Minor, H. General procedure for evaluating amorphous scattering and crystallinity from X-ray diffraction scans of semicrystalline polymers. Polymer 1990, 31, 996–1001. [Google Scholar] [CrossRef]
- Mubarak, Y.A.; Abbadi, F.O.; Tobgy, A.H. Effect of iron oxide nanoparticles on the morphological properties of isotactic polypropylene. J. Appl. Polym. Sci. 2010, 115, 3423–3433. [Google Scholar] [CrossRef]
- Threlfall, T. Structural and Thermodynamic Explanations of Ostwald’s Rule. Org. Process Res. Dev. 2003, 7, 1017–1027. [Google Scholar] [CrossRef]
- Mileva, D.; Androsch, R.; Zhuravlev, E.; Schick, C. Temperature of Melting of the Mesophase of Isotactic Polypropylene. Macromolecules 2009, 42, 7275–7278. [Google Scholar] [CrossRef]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene: III. The equilibrium melting point and the phase diagram. Polymer 1998, 39, 3735–3744. [Google Scholar] [CrossRef]
- Zhang, L.; Van Drongelen, M.; Alfonso, G.C.; Peters, G.W.M. The effect of pressure pulses on isotactic polypropylene crystallization. Eur. Polym. J. 2015, 71, 185–195. [Google Scholar] [CrossRef]
- Yang, S.-G.; Chen, Y.-H.; Deng, B.-W.; Lei, J.; Li, L.; Li, Z.-M. Window of Pressure and Flow To Produce β-Crystals in Isotactic Polypropylene Mixed with β-Nucleating Agent. Macromolecules 2017, 50, 4807–4816. [Google Scholar] [CrossRef]
- Konishi, T.; Nishida, K.; Kanaya, T. Crystallization of Isotactic Polypropylene from Prequenched Mesomorphic Phase. Macromolecules 2006, 39, 8035–8040. [Google Scholar] [CrossRef]
- Ferrero, A.; Ferracini, E.; Mazzavillani, A.; Malta, V. A New X-Ray Study of the Quenched Isotactic Polypropylene Transition by Annealing. J. Macromol. Sci. Phys. Part B 2006, 39, 109–129. [Google Scholar] [CrossRef]
- Fereidoon, A.; Ahangari, M.G.; Saedodin, S. Study of the Nonisothermal Crystallization Kinetics and Melting Behaviors of Polypropylene Reinforced with Single-Walled Carbon Nanotubes Nanocomposite. J. Macromol. Sci. Part B 2008, 48, 25–40. [Google Scholar] [CrossRef]
- Dimeska, A.; Phillips, P.J. High pressure crystallization of random propylene–ethylene copolymers: α–γ Phase diagram. Polymer 2006, 47, 5445–5456. [Google Scholar] [CrossRef]
- Li, J. Multiwalled Carbon Nanotubes Reinforced Polypropylene Composite Material. J. Nanomater. 2017, 2017, 1–5. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Di Girolamo, R.; De Ballesteros, O.R.; Pepe, M.; Tarallo, O.; Malafronte, A. Morphology and Mechanical Properties of the Mesomorphic Form of Isotactic Polypropylene in Stereodefective Polypropylene. Macromolecules 2013, 46, 5202–5214. [Google Scholar] [CrossRef]
- Bikiaris, D. Microstructure and Properties of Polypropylene/Carbon Nanotube Nanocomposites. Materials 2010, 3, 2884–2946. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jia, W.; Dong, B.; Yuan, H.; Su, F.; Wang, Z.; Wang, Y.; Liu, C.; Shen, C.; Shao, C. Structure and Mechanical Properties of Multi-Walled Carbon Nanotubes-Filled Isotactic Polypropylene Composites Treated by Pressurization at Different Rates. Polymers 2019, 11, 1294. https://doi.org/10.3390/polym11081294
Li X, Jia W, Dong B, Yuan H, Su F, Wang Z, Wang Y, Liu C, Shen C, Shao C. Structure and Mechanical Properties of Multi-Walled Carbon Nanotubes-Filled Isotactic Polypropylene Composites Treated by Pressurization at Different Rates. Polymers. 2019; 11(8):1294. https://doi.org/10.3390/polym11081294
Chicago/Turabian StyleLi, Xiaoting, Wenxia Jia, Beibei Dong, Huan Yuan, Fengmei Su, Zhen Wang, Yaming Wang, Chuntai Liu, Changyu Shen, and Chunguang Shao. 2019. "Structure and Mechanical Properties of Multi-Walled Carbon Nanotubes-Filled Isotactic Polypropylene Composites Treated by Pressurization at Different Rates" Polymers 11, no. 8: 1294. https://doi.org/10.3390/polym11081294
APA StyleLi, X., Jia, W., Dong, B., Yuan, H., Su, F., Wang, Z., Wang, Y., Liu, C., Shen, C., & Shao, C. (2019). Structure and Mechanical Properties of Multi-Walled Carbon Nanotubes-Filled Isotactic Polypropylene Composites Treated by Pressurization at Different Rates. Polymers, 11(8), 1294. https://doi.org/10.3390/polym11081294