High-Voltage Sulfolane Plasticized UV-Curable Gel Polymer Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Preparation of GPE
2.3. Characterizations
3. Results and Discussion
3.1. Detecting of UV-Cured GPE
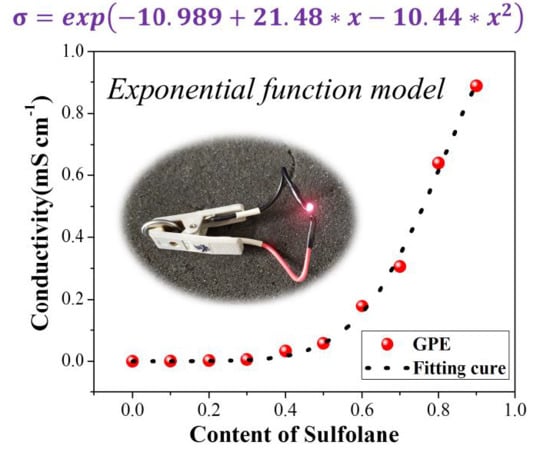
3.2. Effect of Sulfolane Content on GPE
3.3. Lithium Metal Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nitta, N.; Wu, F.X.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Amalraj, S.F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Goodenough, J.B. How we made the li-ion rechargeable battery. Nat. Electron. 2018, 1, 204. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and reality of ni-rich cathode for commercialization. Adv. Energy Mater. 2018, 8, 1702028. [Google Scholar] [CrossRef]
- Lim, J.M.; Hwang, T.; Kim, D.; Park, M.S.; Cho, K.; Cho, M. Intrinsic origins of crack generation in ni-rich LiNi0.8Co0.1Mn0.1O2 layered oxide cathode material. Sci. Rep. 2017, 7, 39669. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.W.; Wang, S.F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Yang, S.B. A material perspective of rechargeable metallic lithium anodes. Adv. Energy Mater. 2018, 8, 1702296. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Xie, Y.; Xu, H.; Jia, J.; Li, J. Developing high-performance lithium metal anode in liquid electrolytes: Challenges and progress. Adv. Mater. 2018, 30, 1706375. [Google Scholar] [CrossRef]
- Cheng, X.L.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H.S. Gel polymer electrolytes for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Liang, S.; Yan, W.; Wu, X.; Zhang, Y.; Zhu, Y.; Wang, H.; Wu, Y. Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance. Solid State Ion. 2018, 318, 2–18. [Google Scholar] [CrossRef]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, K.H.; Cho, S.J.; Park, J.S.; Cho, K.Y.; Lee, C.K.; Lee, S.B.; Shim, J.K.; Lee, S.Y. A shape-deformable and thermally stable solid-state electrolyte based on a plastic crystal composite polymer electrolyte for flexible/safer lithium-ion batteries. J. Mater. Chem. A 2014, 2, 10854–10861. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Ding, F.; Lu, Q.; Liu, J.; Zhang, Q.; Liu, X.; Xu, Q. A novel plastic crystal composite polymer electrolyte with excellent mechanical bendability and electrochemical performance for flexible lithium-ion batteries. Solid State Ion. 2016, 289, 1–8. [Google Scholar] [CrossRef]
- Lin, D.; Yuen, P.Y.; Liu, Y.; Liu, W.; Liu, N.; Dauskardt, R.H.; Cui, Y. A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 2018, 30, 1802661. [Google Scholar] [CrossRef]
- Lv, P.; Li, Y.; Wu, Y.; Liu, G.; Liu, H.; Li, S.; Tang, C.; Mei, J.; Li, Y. Robust succinonitrile-based gel polymer electrolyte for lithium-ion batteries withstanding mechanical folding and high temperature. ACS Appl. Mater. Interfaces 2018, 10, 25384–25392. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.M.; Liang, W.; Shao, Y.; Liu, K.; Nikolov, Z.; Zhu, Y. A superionic conductive, electrochemically stable dual-salt polymer electrolyte. Joule 2018, 2, 1838–1856. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, H.; Li, S.; Zhao, Y.; Mao, L.; Zhao, W.; Li, Y.; Ye, X. Electrochemical performances of a novel high-voltage electrolyte based upon sulfolane and γ-butyrolactone. J. Power Sources 2013, 240, 476–485. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, Y.Y.; Shi, X.M.; Li, B.C.; Xu, X.L.; Zhao, W.; Cui, X.L. Effect of sulfolane on the performance of lithium bis(oxalato)borate-based electrolytes for advanced lithium ion batteries. Electrochim. Acta 2012, 65, 221–227. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Tan, G.X.; Wang, Y.J.; Li, J.; Zhang, S.J. Synthesis and characterization of injectable photocrosslinking poly (ethylene glycol) diacrylate based hydrogels. Polym. Bull. 2008, 61, 91–98. [Google Scholar] [CrossRef]
- Fan, W.; Li, N.W.; Zhang, X.; Zhao, S.; Cao, R.; Yin, Y.; Xing, Y.; Wang, J.; Guo, Y.G.; Li, C. A dual-salt gel polymer electrolyte with 3d cross-linked polymer network for dendrite-free lithium metal batteries. Adv. Sci. 2018, 5, 1800559. [Google Scholar] [CrossRef]
- Anderson, O.; Stuart, D. Calculation of activation energy of ionic conductivity in silica glasses by classical methods. J. Am. Ceram. Soc. 1954, 37, 573–580. [Google Scholar] [CrossRef]
- Peters, A.; Korte, C.; Hesse, D.; Zakharov, N.; Janek, J. Ionic conductivity and activation energy for oxygen ion transport in superlattices—the multilayer system CSZ (ZrO2+ CaO)/Al2O3. Solid State Ion. 2007, 178, 67–76. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wei, C.; Ding, W.; Zou, L.; Gong, Y.; Liu, Y.; Zang, L.; Xu, X. High-Voltage Sulfolane Plasticized UV-Curable Gel Polymer Electrolyte. Polymers 2019, 11, 1306. https://doi.org/10.3390/polym11081306
Wang S, Wei C, Ding W, Zou L, Gong Y, Liu Y, Zang L, Xu X. High-Voltage Sulfolane Plasticized UV-Curable Gel Polymer Electrolyte. Polymers. 2019; 11(8):1306. https://doi.org/10.3390/polym11081306
Chicago/Turabian StyleWang, Shiqi, Chun Wei, Wenwen Ding, Linmin Zou, Yongyang Gong, Yuanli Liu, Limin Zang, and Xu Xu. 2019. "High-Voltage Sulfolane Plasticized UV-Curable Gel Polymer Electrolyte" Polymers 11, no. 8: 1306. https://doi.org/10.3390/polym11081306
APA StyleWang, S., Wei, C., Ding, W., Zou, L., Gong, Y., Liu, Y., Zang, L., & Xu, X. (2019). High-Voltage Sulfolane Plasticized UV-Curable Gel Polymer Electrolyte. Polymers, 11(8), 1306. https://doi.org/10.3390/polym11081306