Thioetherimide-Modified Cyanate Ester Resin with Better Molding Performance for Glass Fiber Reinforced Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Long-Chain Thioether BMI
2.3. Preparation of Glass Fiber Cloth Prepreg
2.4. Preparation of Composites
2.5. Characterization
3. Results and Discussion
3.1. Infrared Spectrum of Synthetic Resin
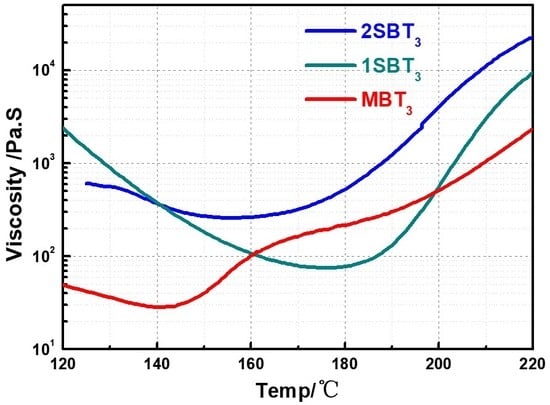
3.2. Rheological Properties of Resins
3.3. Thermogravimetric Analysis
3.4. Hygroscopicity Analysis
3.5. Mechanical Properties of Composites
3.6. Dimensional Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamerton, I.; Hay, J.N. Recent technological developments in cyanate ester resins. High Perform. Polym. 1998, 10, 163–174. [Google Scholar] [CrossRef]
- Liu, J.; Fan, W.; Lu, G.; Zhou, D.; Wang, Z.; Yan, J. Semi-Interpenetrating Polymer Networks Based on Cyanate Ester and Highly Soluble Thermoplastic Polyimide. Polymers 2019, 11, 862. [Google Scholar] [CrossRef]
- Liu, P.; Qu, C.; Wang, D.; Zhao, M.; Liu, C. High-performance bismaleimide resins with low cure temperature for resin transfer molding process. High Perform. Polym. 2017, 29, 298–304. [Google Scholar] [CrossRef]
- Crawford, A.O.; Cavalli, G.; Howlin, B.J.; Hamerton, I. Investigation of structure property relationships in liquid processible, solvent free, thermally stable bismaleimide-triazine (BT) resins. React. Funct. Polym. 2016, 102, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Guenthner, A.J.; Yandek, G.R.; Wright, M.E.; Petteys, B.J.; Quintana, R.; Connor, D.; Gilardi, R.D.; Marchant, D. A New Silicon-Containing Bis(cyanate) Ester Resin with Improved Thermal Oxidation and Moisture Resistance. Macromolecules 2006, 39, 6046–6053. [Google Scholar] [CrossRef]
- Hamerton, I.; Hay, J.N. Recent developments in the chemistry of cyanate esters †. Polym. Int. 1998, 47, 465–473. [Google Scholar] [CrossRef]
- Tang, Y.-S.; Kong, J.; Gu, J.-W.; Liang, G.-Z. Reinforced cyanate ester resins with carbon nanotubes: Surface modification, reaction activity and mechanical properties analyses. Polym.-Plast. Technol. Eng. 2009, 48, 359–366. [Google Scholar] [CrossRef]
- Badrinarayanan, P.; Rogalski, M.K.; Kessler, M.R. Carbon fiber-reinforced cyanate ester/nano-ZrW2O8 composites with tailored thermal expansion. ACS Appl. Mat. Interfaces 2011, 4, 510–517. [Google Scholar] [CrossRef]
- Wu, H.; Kessler, M.R. Multifunctional cyanate ester nanocomposites reinforced by hexagonal boron nitride after noncovalent biomimetic functionalization. ACS Appl. Mat. Interfaces 2015, 7, 5915–5926. [Google Scholar] [CrossRef]
- Goertzen, W.K.; Kessler, M. Thermal and mechanical evaluation of cyanate ester composites with low-temperature processability. Composites Part A 2007, 38, 779–784. [Google Scholar] [CrossRef]
- Hamerton, I.; Barton, J.M.; Chaplin, A.; Howlin, B.J.; Shaw, S.J. The development of novel functionalised aryl cyanate esters. Part 2. Mechanical properties of the polymers and composites. Polymer 2001, 42, 2307–2319. [Google Scholar] [CrossRef]
- Karad, S.K.; Jones, F.R.; Attwood, D. Moisture absorption by cyanate ester modified epoxy resin matrices. Part I. Effect of spiking parameters. Polymer 2002, 43, 5209–5218. [Google Scholar] [CrossRef]
- Iijima, T.; Katsurayama, S.; Fukuda, W.; Tomoi, M. Modification of cyanate ester resin by poly (ethylene phthalate) and related copolyesters. J. Appl. Polym. Sci. 2000, 76, 208–219. [Google Scholar] [CrossRef]
- Iredale, R.J.; Ward, C.; Hamerton, I. Modern advances in bismaleimide resin technology: A 21st century perspective on the chemistry of addition polyimides. Prog. Polym. Sci. 2017, 69, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Ding, L.; Li, W.; Ma, G.; Bai, H.; Li, L. Hyper-cross-linked organic microporous polymers based on alternating copolymerization of bismaleimide. ACS Macro Lett. 2016, 5, 377–381. [Google Scholar] [CrossRef]
- Hou, H.; Xu, W.; Ding, Y. The recent progress on high-performance polymer nanofibers by electrospinning. J. Jiangxi Norm. Univ. (Nat. Sci.) 2018, 42, 551–564. [Google Scholar] [CrossRef]
- Xu, M.; Lei, Y.; Ren, D.; Chen, L.; Li, K.; Liu, X. Thermal stability of allyl-functional phthalonitriles-containing benzoxazine/bismaleimide copolymers and their improved mechanical properties. Polymers 2018, 10, 596. [Google Scholar] [CrossRef]
- Ding, C.; Fang, H.; Duan, G.; Zou, Y.; Chen, S.; Hou, H. Investigating the draw ratio and velocity of an electrically charged liquid jet during electrospinning. RSC Adv. 2019, 9, 13608–13613. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Ishige, R.; Ando, S. Enhancement of thermal diffusivity in phase-separated bismaleimide/poly (ether imide) composite films containing needle-shaped ZnO particles. Polymers 2017, 9, 263. [Google Scholar] [CrossRef]
- Kuang, J.; Zheng, N.; Liu, C.; Zheng, Y. Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers. e-Polym. 2019, 19, 355–364. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Gao, L.; Ding, M. Polyimides from isomeric diphenylthioether dianhydrides. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 959–967. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Wu, H.; Lin, Z.; Zhang, Z.; Wang, R.; Li, B.; Kang, F.; Shi, L.; Wong, C.P. Future paper based printed circuit boards for green electronics: Fabrication and life cycle assessment. Energy Environ. Sci. 2014, 7, 3674–3682. [Google Scholar] [CrossRef]
- Tang, Y.; Dong, W.; Tang, L.; Zhang, Y.; Kong, J.; Gu, J. Fabrication and investigations on the polydopamine/KH-560 functionalized PBO fibers/cyanate ester wave-transparent composites. Compos. Commun. 2018, 8, 36–41. [Google Scholar] [CrossRef]
- Liu, J.; Meng, X.; Yang, H.; Wang, Z.; Fan, W. Method for producing bismaleimide resin modified cyanate initial rinse material. Chinese Patents CN200710300340.6, 7 December 2017. [Google Scholar]
- Yao, K.; Chen, J.; Li, P.; Duan, G.; Hou, H. Robust strong electrospun polyimide composite nanofibers from a ternary polyamic acid blend. Compos. Commun. 2019, 15, 92–95. [Google Scholar] [CrossRef]
- Chen, Y.; Sui, L.; Fang, H.; Ding, C.; Li, Z.; Jiang, S.; Hou, H. Superior mechanical enhancement of epoxy composites reinforced by polyimide nanofibers via a vacuum-assisted hot-pressing. Compos. Sci. Technol. 2019, 174, 20–26. [Google Scholar] [CrossRef]
- Huang, B.; Wang, X.; Fang, H.; Jiang, S.; Hou, H. Mechanically strong sulfonated polybenzimidazole PEMs with enhanced proton conductivity. Mater. Lett. 2019, 234, 354–356. [Google Scholar] [CrossRef]
- Liao, X.; Ye, W.; Chen, L.; Jiang, S.; Wang, G.; Zhang, L.; Hou, H. Flexible hdC-G reinforced polyimide composites with high dielectric permittivity. Compos. Part A 2017, 101, 50–58. [Google Scholar] [CrossRef]
- Sava, M.; Vlad, S. Synthesis and properties of some polyaminobis maleimides thermal and mechanical characterization. e-Polym. 2008, 8. no. 046. [Google Scholar] [CrossRef]
- Bao, L.-R.; Yee, A.F. Effect of temperature on moisture absorption in a bismaleimide resin and its carbon fiber composites. Polymer 2002, 43, 3987–3997. [Google Scholar] [CrossRef]
- Bell, V.L.; Young, P.R. Isomeric bismaleimides and polyaspartimides. J. Polym. Sci. Part. A Polym. Chem. 1986, 24, 2647–2655. [Google Scholar] [CrossRef]
- Dix, L.R.; Ebdon, J.R.; Flint, N.J.; Hodge, P.; O’Dell, R. Chain extension and crosslinking of telechelic oligomers—I. Michael additions of bisamines to bismaleimides and bis (acetylene ketone) s. Eur. Polym. J. 1995, 31, 647–652. [Google Scholar] [CrossRef]






| Code | T5% (°C) | Code | T5% (°C) | Code | T5% (°C) |
|---|---|---|---|---|---|
| 1SBT1 | 395 | 2SBT1 | 392 | MBT1 | 404 |
| 1SBT2 | 405 | 2SBT2 | 406 | MBT2 | 407 |
| 1SBT3 | 414 | 2SBT3 | 415 | MBT3 | 413 |
| Entry | GMBT1 | GMBT2 | GMBT3 | G1SBT1 | G1SBT2 | G1SBT3 | G2SBT1 | G2SBT2 | G2SBT3 |
|---|---|---|---|---|---|---|---|---|---|
| Moisture absorption (%) | 2.8 ± 0.2 | 3.5 ± 0.4 | 3.7 ± 0.3 | 1.9 ± 0.2 | 1.5 ± 0.1 | 1.4 ± 0.1 | 2.0 ± 0.2 | 1.6 ± 0.1 | 1.5 ± 0.1 |
| Code | Test Temperature (°C) | Tensile Strength (MPa) | Retention Rate (%) | Elongation at Break (%) | Flexural Strength (MPa) | Retention Rate (%) |
|---|---|---|---|---|---|---|
| G1SBT1 | 20 | 366 ± 35 | 85 | 8.2 ± 0.7 | 576 ± 56 | 47 |
| 200 | 311 ± 30 | 10.2 ± 1.2 | 270 ± 38 | |||
| G1SBT2 | 20 | 384 ± 46 | 84 | 8.4 ± 0.9 | 604 ± 59 | 46 |
| 200 | 323 ± 31 | 10.4 ± 1.0 | 278 ± 26 | |||
| G1SBT3 | 20 | 431 ± 55 | 70 | 8.7 ± 0.4 | 614 ± 72 | 45 |
| 200 | 331 ± 42 | 10.8 ± 0.5 | 278 ± 16 |
| Code | Test Temperature (°C) | Tensile Strength (MPa) | Retention Rate (%) | Elongation at Break (%) | Flexural Strength (MPa) | Retention Rate (%) |
|---|---|---|---|---|---|---|
| G2SBT1 | 20 | 373 ± 42 | 85 | 8.9 ± 0.9 | 585 ± 67 | 48 |
| 200 | 317 ± 35 | 11.2 ± 1.3 | 279 ± 29 | |||
| G2SBT2 | 20 | 419 ± 43 | 84 | 9.1 ± 0.7 | 647 ± 53 | 47 |
| 200 | 352 ± 27 | 12.4 ± 1. 4 | 307 ± 42 | |||
| G2SBT3 | 20 | 439 ± 32 | 71 | 9.2 ± 0.6 | 657 ± 68 | 46 |
| 200 | 382 ± 36 | 12.5 ± 1.5 | 307 ± 32 |
| Entry | GMBT1 | GMBT2 | GMBT3 | G1SBT1 | G1SBT2 | G1SBT3 | G2SBT1 | G2SBT2 | G2SBT3 |
|---|---|---|---|---|---|---|---|---|---|
| CTE (×10−6m/°C) | 17.1 | 16.6 | 15.7 | 17.3 | 17.8 | 18.1 | 17.5 | 18.3 | 18.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, P.; Dai, C.; Jiang, S. Thioetherimide-Modified Cyanate Ester Resin with Better Molding Performance for Glass Fiber Reinforced Composites. Polymers 2019, 11, 1458. https://doi.org/10.3390/polym11091458
Ma P, Dai C, Jiang S. Thioetherimide-Modified Cyanate Ester Resin with Better Molding Performance for Glass Fiber Reinforced Composites. Polymers. 2019; 11(9):1458. https://doi.org/10.3390/polym11091458
Chicago/Turabian StyleMa, Pengchang, Chuntao Dai, and Shaohua Jiang. 2019. "Thioetherimide-Modified Cyanate Ester Resin with Better Molding Performance for Glass Fiber Reinforced Composites" Polymers 11, no. 9: 1458. https://doi.org/10.3390/polym11091458
APA StyleMa, P., Dai, C., & Jiang, S. (2019). Thioetherimide-Modified Cyanate Ester Resin with Better Molding Performance for Glass Fiber Reinforced Composites. Polymers, 11(9), 1458. https://doi.org/10.3390/polym11091458