Polyamide 6/Poly(vinylidene fluoride) Blend-Based Nanocomposites with Enhanced Rigidity: Selective Localization of Carbon Nanotube and Organoclay
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Characterizations
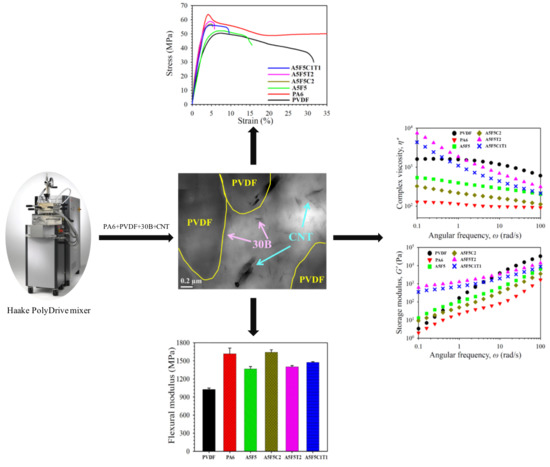
3. Results and Discussion
3.1. Phase Morphology and Selective Localization of Nanofillers
3.2. Crystal Structure
3.3. Crystallization and Melting Behavior
3.4. Mechanical Properties
3.5. Rheological Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ho, J.C.; Wei, K.H. Induced crystal transformation in blends of polyamide 6 and liquid crystalline copolyester. Macromolecules 2000, 33, 5181–5186. [Google Scholar] [CrossRef]
- Miyasaka, K.; Ishikawa, M. Effects of temperature and water on the crystalline transition of nylon 6 caused by stretching in the chain direction. J. Polym. Sci. Part A-2 1968, 6, 1317–1329. [Google Scholar] [CrossRef]
- Dusek, K.; Patterson, D. Transition in swollen polymer networks induced by intramolecular condensation. J. Polym. Sci. Part A-2 1968, 6, 1209–1216. [Google Scholar] [CrossRef]
- Murthy, N.S. Metastable crystalline phases in nylon 6. Polym. Commun. 1991, 32, 301–305. [Google Scholar]
- Li, J.; Fang, Z.; Tong, L.; Gu, A.; Liu, F. Polymorphism of nylon-6 in multiwalled carbon nanotubes/nylon-6 composites. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1499–1512. [Google Scholar] [CrossRef]
- Chiu, F.C. Poly(vinylidene fluoride)/polycarbonate blend-based nanocomposites with enhanced rigidity-selective localization of carbon nanofillers and organoclay. Polym. Test. 2017, 62, 115–123. [Google Scholar] [CrossRef]
- Elnabawy, E.; Hassanain, A.H.; Shehata, N.; Popelka, A.; Nair, R.; Yousef, S.; Kandas, I. Piezoelastic PVDF/TPU nanofibrous composite membrane: Fabrication and characterization. Polymers 2019, 11, 1634. [Google Scholar] [CrossRef] [Green Version]
- Lovinger, A.J. Crystallization of the phase of poly(vinylidene fluoride) from the melt. Polymer 1981, 22, 412–413. [Google Scholar] [CrossRef]
- Mohajir, B.E.E.; Heymans, N. Changes in structural and mechanical behaviour of PVDF with processing and thermomechanical treatments. 1. change in structure. Polymer 2001, 42, 5661–5667. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, T.; Huang, T.; Zhang, N.; Wang, Y.; Yang, J. Fabrication of high-k poly(vinylidene fluoride)/nylon 6/carbon nanotube nanocomposites through selective localization of carbon nanotubes in blends. Polym. Int. 2017, 66, 604–611. [Google Scholar] [CrossRef]
- Chiu, F.C.; Lai, S.M.; Chen, Y.L.; Lee, T.H. Investigation on the polyamide 6/organoclay nanocomposites with or without a maleated polyolefin elastomer as a toughener. Polymer 2005, 46, 11600–11609. [Google Scholar] [CrossRef]
- Li, J.; Tong, L.; Fang, Z.; Gu, A.; Xu, Z. Thermal degradation behavior of multi-walled carbon nanotubes/polyamide 6 composites. Polym. Degrad. Stab. 2006, 91, 2046–2052. [Google Scholar] [CrossRef]
- Pramoda, K.; Mohamed, A.; Phang, I.Y.; Liu, T. Crystal transformation and thermomechanical properties of poly(vinylidene fluoride)/clay nanocomposites. Polym. Int. 2005, 54, 226–232. [Google Scholar] [CrossRef]
- Chiu, F.C. Comparisons of phase morphology and physical properties of PVDF nanocomposites filled with organoclay and/or multi-walled carbon nanotubes. Mater. Chem. Phys. 2014, 143, 681–692. [Google Scholar] [CrossRef]
- Mahmood, N.; Islam, M.; Hameed, A.; Saeed, S. Polyamide 6/multiwalled carbon nanotubes nanocomposites with modified morphology and thermal properties. Polymers 2013, 5, 1380–1391. [Google Scholar] [CrossRef]
- Du, F.P.; Qiao, X.; Wu, Y.G.; Fu, P.; Liu, S.P.; Zhang, Y.F.; Wang, Q.Y. Fabrication of porous polyvinylidene fluoride/multi-walled carbon nanotube nanocomposites and their enhanced thermoelectric performance. Polymers 2018, 10, 797. [Google Scholar] [CrossRef] [Green Version]
- Otaegi, I.; Aramburu, N.; Müller, A.J.; Gonzalo, G.E. Novel biobased polyamide 410/polyamide 6/CNT nanocomposites. Polymers 2018, 10, 986. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Ko, K.; Youk, J.; Lim, D.; Jeong, W. Preparation and properties of carbon fiber/carbon nanotube wet-laid composites. Polymers 2019, 11, 1597. [Google Scholar] [CrossRef] [Green Version]
- Huitric, J.; Ville, J.; Mederic, P.; Aubry, T. Solid-state morphology, structure, and tensile properties of polyethylene/polyamide/nanoclay blends: Effect of clay fraction. Polym. Test. 2017, 58, 96–103. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chen, C.C.; Chen, Y.J. Binary and ternary nanocomposites based on PVDF, PMMA, and PVDF/PMMA blends: Polymorphism, thermal, and rheological properties. J. Polym. Res. 2014, 21, 378–389. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chen, Y.J. Evaluation of thermal, mechanical, and electrical properties of PVDF/GNP binary and PVDF/PMMA/GNP ternary nanocomposites. Compos. Part A Appl. Sci. Manuf. 2015, 68, 62–71. [Google Scholar] [CrossRef]
- Li, L.; Ruan, W.H.; Zhang, M.Q.; Rong, M.Z. Studies on the selective localization of multi-walled carbon nanotubes in blends of poly(vinylidene fluoride) and polycaprolactone. Polym. Lett. 2015, 9, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Shimizu, H. Conductive PVDF/PA6/CNTs nanocomposites fabricated by dual formation of cocontinuous and nanodispersion structures. Macromolecules 2008, 41, 5339–5344. [Google Scholar] [CrossRef]
- Chen, J.; Lu, H.Y.; Yang, J.H.; Wanga, Y.; Zheng, X.T.; Zhang, C.L.; Yuan, G.P. Effect of organoclay on morphology and electrical conductivity of PC/PVDF/CNT blend composites. Compos. Sci. Technol. 2014, 94, 30–38. [Google Scholar] [CrossRef]
- Chiu, F.C. Halloysite nanotube- and organoclay-filled biodegradable poly(butylene succinate-co-adipate)/maleated polyethylene blend based nanocomposites with enhanced rigidity. Compos. Part B Eng. 2017, 110, 193–203. [Google Scholar] [CrossRef]
- Gomari, S.; Ghasemi, I.; Karrabi, M.; Azizi, H. Organoclay localization in polyamide 6/ethylene-butene copolymer grafted maleic anhydride blends: The effect of different types of organoclay. J. Polym. Res. 2012, 19, 9769–9779. [Google Scholar] [CrossRef]
- Zhao, X.D.; Zhao, J.; Cao, J.P.; Wang, D.R.; Hub, G.H.; Chen, F.H.; Dang, Z.M. Effect of the selective localization of carbon nanotubes in polystyrene/poly(vinylidene fluoride) blends on their dielectric, thermal, and mechanical properties. Mater. Des. 2014, 56, 807–815. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Naderi, G.; Dubois, C. Rheological and morphological properties of PA6/ECO nanocomposites. Rheol. Acta 2010, 49, 1015–1027. [Google Scholar] [CrossRef]
- Dharaiya, D.; Jana, S.C. Thermal decomposition of alkyl ammonium ions and its effects on surface polarity of organically treated nanoclay. Polymer 2005, 46, 10139–10147. [Google Scholar] [CrossRef]
- Nuriel, S.; Liu, L.; Barber, A.H.; Wagner, H.D. Direct measurement of multiwall nanotube surface tension. Chem. Phys. Lett. 2005, 404, 263–266. [Google Scholar] [CrossRef]
- Vo, L.T.; Giannelis, E.P. Compatibilizing poly(vinylidene fluoride)/nylon-6 blends with nanoclay. Macromolecules 2007, 40, 8271–8276. [Google Scholar] [CrossRef]
- Li, Y.; Iwakura, Y.; Shimizu, H. Crystal form and phase structure of poly(vinylidene fluoride)/polyamide 11/clay nanocomposites by high-shear processing. J. Nanosci. Nanotechnol. 2008, 8, 1714–1720. [Google Scholar] [PubMed]
- Wang, B.; Wang, W.; Wang, H.; Hu, G. Isothermal crystallization kinetics and melting behavior of in situ compatibilized polyamide 6/polyethylene-octene blends. J. Polym. Res. 2010, 17, 429–437. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Jiang, S.L.; Yu, Y.; Zeng, Y.K.; Zhang, G.Z.; Zhang, Q.F.; He, J.G. Crystallization behavior and phase-transformation mechanism with the use of graphite nanosheets in poly(vinylidene fluoride) nanocomposites. J. Appl. Polym. Sci. 2012, 125, 314–319. [Google Scholar] [CrossRef]
- Priya, L.; Jog, J.P. Polymorphism in intercalated poly(vinylidene fluoride)/clay nanocomposites. J. Appl. Polym. Sci. 2003, 89, 2036–2040. [Google Scholar] [CrossRef]
- Virtanen, S.; Vartianen, J.; Setälä, H.; Tammelinb, T.; Vuotic, S. Modified nanofibrillated cellulose-polyvinyl alcohol films with improved mechanical performance. RSC Adv. 2014, 4, 11343–11350. [Google Scholar] [CrossRef] [Green Version]
- Klata, E.; Van de Velde, K.; Krucin’ska, I. DSC investigations of polyamide 6 in hybrid GF/PA 6 yarns and composites. Polym. Test. 2003, 22, 929–937. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Weeks, J.J. Melting process and the equilibrium melting temperature of polychlorotrifluoroethylene. J. Res. Natl. Bur. Stand. A Phys. Chem. 1962, 66A, 13–28. [Google Scholar] [CrossRef]
- Liu, Z.H.; Maréchal, P.; Jérôme, R. Blends of poly(vinylidene fluoride) with polyamide 6: Interfacial adhesion, morphology and mechanical properties. Polymer 1998, 39, 1779–1785. [Google Scholar] [CrossRef] [Green Version]







| Material | Total Surface Free Energy | Temperature Coefficient | Dispersive Part | Polar Part |
|---|---|---|---|---|
| γ (mJm−2) | −dγdT−1 (mJm−2*K) | |||
| PVDF [27] | 30.3 | 0 | 23.3 | 7.0 |
| PA6 [28] | 37.7 | 0.065 [26] | 27.1 | 10.6 |
| 30B [29] | 36.0 | 0.1 [26] | 23.0 | 13.0 |
| CNT [30] | 45.3 | 0 | 18.4 | 26.9 |
| Material | Interfacial Tension (mMm−1) | Wetting Coefficient, | Location Prediction | ||
|---|---|---|---|---|---|
| Harmonic Mean Equation | Geometric Mean Equation | Harmonic Mean Equation | Geometric Mean Equation | ||
| 1.0 | 0.5 | ||||
| 1.8 | 0.9 | ||||
| 0.6 | 0.3 | ||||
| 1.2 | 1.2 | PA6 | |||
| 12.3 | 6.7 | ||||
| 8.8 | 4.6 | ||||
| 3.4 | 4.2 | PA6 | |||
| Samples | Properties | |||||
|---|---|---|---|---|---|---|
| TcPVDF (°C) a | TcPA6 (°C) a | χcPVDF (%) a | χcPA6 (%) a | TmPVDF (°C) b | TmPA6 (°C) b | |
| PVDF | 139.0 | -- | 46.2 | -- | 172.0 | -- |
| PA6 | -- | 194.1 | -- | 27.5 | -- | 220.9 |
| A5F5 | 126.1/137.1 | 192.8 | 50.4 | 26.3 | 169.9 | 219.4 |
| A5F5C2 | 136.3 | 190.8 | 42.6 | 27.0 | 170.8 | 215.2 |
| A5F5T2 | 125.3/133.9 | 197.8 | 45.9 | 28.3 | 169.9 | 220.7 |
| A5F5C1T1 | 137.1 | 197.2 | 37.6 | 28.8 | 170.2 | 220.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-M.; Behera, K.; Yadav, M.; Chiu, F.-C. Polyamide 6/Poly(vinylidene fluoride) Blend-Based Nanocomposites with Enhanced Rigidity: Selective Localization of Carbon Nanotube and Organoclay. Polymers 2020, 12, 184. https://doi.org/10.3390/polym12010184
Lin H-M, Behera K, Yadav M, Chiu F-C. Polyamide 6/Poly(vinylidene fluoride) Blend-Based Nanocomposites with Enhanced Rigidity: Selective Localization of Carbon Nanotube and Organoclay. Polymers. 2020; 12(1):184. https://doi.org/10.3390/polym12010184
Chicago/Turabian StyleLin, Hung-Ming, Kartik Behera, Mithilesh Yadav, and Fang-Chyou Chiu. 2020. "Polyamide 6/Poly(vinylidene fluoride) Blend-Based Nanocomposites with Enhanced Rigidity: Selective Localization of Carbon Nanotube and Organoclay" Polymers 12, no. 1: 184. https://doi.org/10.3390/polym12010184
APA StyleLin, H. -M., Behera, K., Yadav, M., & Chiu, F. -C. (2020). Polyamide 6/Poly(vinylidene fluoride) Blend-Based Nanocomposites with Enhanced Rigidity: Selective Localization of Carbon Nanotube and Organoclay. Polymers, 12(1), 184. https://doi.org/10.3390/polym12010184