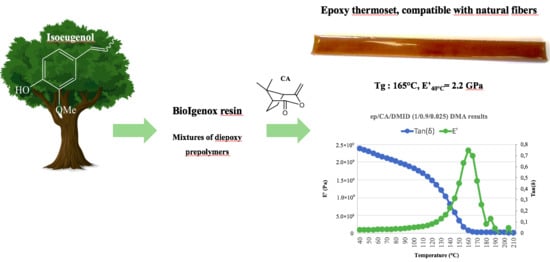
New Eco-Friendly Synthesized Thermosets from Isoeugenol-Based Epoxy Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Characterizations and Instruments
First Step: Synthesis of BioIgenox
Second Step: Synthesis of BioIgenox
2.2.2. Curing Reaction
3. Results and Discussion
3.1. Reactional Mechanism and Characterization of BioIgenol Mixture
3.2. Formation, Characterization and Curing of BioIgenox Mixture
3.3. Properties and Characterization of BioIgenox Based Thermosets
3.3.1. Effect of the Epoxide Function/Anhydride Ratio on the Thermoset’s Properties
3.3.2. Effect of the Curing Agent on the Properties of the Thermoset
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- European Chemical Agency. Commitee for Risk Assesment RAC Proposing Hamonised Classification and Labelling at EU Level of Bisphenol A; European Chemical Agency: Helsinki, Finland, 2014.
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An Endocrine and Metabolic Disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef]
- Wang, X.-L.; Chen, L.; Wu, J.-N.; Fu, T.; Wang, Y.-Z. Flame-Retardant Pressure-Sensitive Adhesives Derived from Epoxidized Soybean Oil and Phosphorus-Containing Dicarboxylic Acids. ACS Sustain. Chem. Eng. 2017, 5, 3353–3361. [Google Scholar] [CrossRef]
- Richaud, E.; Guinault, A.; Baiz, S.; Nizeyimana, F. Epoxidized Linseed Oils Based Networks. Case of Thermal Degradation. Polym. Degrad. Stab. 2019, 166, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Upton, B.M.; Kasko, A.M. Strategies for the Conversion of Lignin to High-Value Polymeric Materials: Review and Perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef]
- Jung, J.Y.; Park, C.-H.; Lee, E.Y. Epoxidation of Methanol-Soluble Kraft Lignin for Lignin-Derived Epoxy Resin and Its Usage in the Preparation of Biopolyester. J. Wood Chem. Technol. 2017, 37, 433–442. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Huang, X.; Whelton, A.J.; Abu-Omar, M.M. Formaldehyde-Free Method for Incorporating Lignin into Epoxy Thermosets. ACS Sustain. Chem. Eng. 2018, 6, 10628–10636. [Google Scholar] [CrossRef]
- Van de Pas, D.J.; Torr, K.M. Biobased Epoxy Resins from Deconstructed Native Softwood Lignin. Biomacromolecules 2017, 18, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Savonnet, E.; Grau, E.; Grelier, S.; Defoort, B.; Cramail, H. Divanillin-Based Epoxy Precursors as DGEBA Substitutes for Biobased Epoxy Thermosets. ACS Sustain. Chem. Eng. 2018, 6, 11008–11017. [Google Scholar] [CrossRef]
- Mora, A.-S.; Tayouo, R.; Boutevin, B.; David, G.; Caillol, S. Vanillin-Derived Amines for Bio-Based Thermosets. Green Chem. 2018, 20, 4075–4084. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkita, T. Fully Biobased Epoxy Resin Systems Composed of a Vanillin-Derived Epoxy Resin and Renewable Phenolic Hardeners. Eur. Polym. J. 2017, 92, 165–173. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J. High-Performance Biobased Epoxy Derived from Rosin. Polym. Int. 2010. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhang, P.; Wolcott, M.; Zhang, J. Use of Eugenol and Rosin as Feedstocks for Biobased Epoxy Resins and Study of Curing and Performance Properties: Use of Eugenol and Rosin as Feedstocks. Polym. Int. 2014, 63, 760–765. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, L.; Zhang, Y.; Liu, Q.; Ru, C.; Zhang, W.; Zhao, C. Novel Biobased Epoxy Resin Thermosets Derived from Eugenol and Vanillin. Polym. Degrad. Stab. 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Faye, I.; Decostanzi, M.; Ecochard, Y.; Caillol, S. Eugenol Bio-Based Epoxy Thermosets: From Cloves to Applied Materials. Green Chem. 2017, 19, 5236–5242. [Google Scholar] [CrossRef]
- Miao, J.-T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Biobased Heat Resistant Epoxy Resin with Extremely High Biomass Content from 2,5-Furandicarboxylic Acid and Eugenol. ACS Sustain. Chem. Eng. 2017, 5, 7003–7011. [Google Scholar] [CrossRef]
- François, C.; Pourchet, S.; Boni, G.; Fontaine, S.; Gaillard, Y.; Placet, V.; Galkin, M.V.; Orebom, A.; Samec, J.; Plasseraud, L. Diglycidylether of Iso-Eugenol: A Suitable Lignin-Derived Synthon for Epoxy Thermoset Applications. RSC Adv. 2016, 6, 68732–68738. [Google Scholar] [CrossRef]
- François, C.; Pourchet, S.; Boni, G.; Rautiainen, S.; Samec, J.; Fournier, L.; Robert, C.; Thomas, C.M.; Fontaine, S.; Gaillard, Y.; et al. Design and Synthesis of Biobased Epoxy Thermosets from Biorenewable Resources. C. R. Chim. 2017, 20, 1006–1016. [Google Scholar] [CrossRef]
- Pourchet, S.; Sonnier, R.; Ben-Abdelkader, M.; Gaillard, Y.; Ruiz, Q.; Placet, V.; Plasseraud, L.; Boni, G. New Reactive Isoeugenol Based Phosphate Flame Retardant: Toward Green Epoxy Resins. ACS Sustain. Chem. Eng. 2019, 7, 14074–14088. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Abu-Omar, M.M. Renewable Epoxy Networks Derived from Lignin-Based Monomers: Effect of Cross-Linking Density. ACS Sustain. Chem. Eng. 2016, 4, 6082–6089. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 7th ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Menard, K.P.; Menard, N.R. Encyclopedia of Polymer Science and Technology, 1st ed.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- Menard, K.P. Dynamic Mechanical Analysis: A Practical Introduction; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Amariutei, O.A.; Ramsdale-Capper, R.; Correa Álvarez, M.; Chan, L.K.Y.; Foreman, J.P. Modelling the Properties of a Difunctional Epoxy Resin Cured with Aromatic Diamine Isomers. Polymer 2018, 156, 203–213. [Google Scholar] [CrossRef]
- Pandini, S.; Bignotti, F.; Baldi, F.; Sartore, L.; Consolati, G.; Panzarasa, G. Thermomechanical and Large Deformation Behaviors of Antiplasticized Epoxy Resins: Effect of Material Formulation and Network Architecture. Polym. Eng. Sci. 2017, 57, 553–565. [Google Scholar] [CrossRef]
- Palmese, G.R.; McCullough, R.L. Effect of Epoxy–Amine Stoichiometry on Cured Resin Material Properties. J. Appl. Polym. Sci. 1992, 46, 1863–1873. [Google Scholar] [CrossRef]
- Henriques, I.R.; Borges, L.A.; Costa, M.F.; Soares, B.G.; Castello, D.A. Comparisons of Complex Modulus Provided by Different DMA. Polym. Test. 2018, 72, 394–406. [Google Scholar] [CrossRef]











| Epoxide Function/Anhydride Molar Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1/0.5 | 1/0.6 | 1/0.7 | 1/0.8 | 1/0.9 | 1/1 | |||
| BI * (mg) | 2.64 | 2.44 | 2.63 | 3.03 | 2.94 | 2.84 | 2.79 | 2.73 |
| CA (mg) | 1.57 | 1.72 | 2.16 | 2.86 | 3.01 | 3.26 | ||
| HHPA (mg) | 3.37 | 2.54 | ||||||
| E′ Max Derivative | Max E″ | Max Tan(δ) | Tg DSC | |
|---|---|---|---|---|
| ep/CA | (°C) | °C | °C | Ramp 10 °C/min (°C) |
| 1/0.5 | 70 | 75 | 90 | 80 |
| 1/0.6 | 85 | 95 | 115 | 105 |
| 1/0.7 | 110 | 115 | 130 | 120 |
| 1/0.8 | 110 | 110 | 130 | 145 |
| 1/0.9 | 140 | 145 | 165 | 150 |
| 1/1 | 145 | 145 | 160 | 155 |
| E′ at 40 °C | Max Tan(δ) | Crosslinking Density | Swelling | ||
|---|---|---|---|---|---|
| ep/CA | GPa | °C * | Amplitude | mol cm−3 | |
| 1/0.5 | 2.57 | 90 | 2.11 | 0.70 | 180 |
| 1/0.6 | 2.62 | 115 | 1.29 | 2.04 | 79 |
| 1/0.7 | 2.60 | 130 | 1.23 | 2.43 | 54 |
| 1/0.8 | 2.41 | 130 | 0.84 | 3.35 | 55 |
| 1/0.9 | 2.23 | 165 | 0.75 | 3.66 | 36 |
| 1/1 | 2.27 | 160 | 0.84 | 4.58 | 35 |
| Mean Value ± Standard Deviation | Modulus | Ultimate Stress | Strain at Failure |
|---|---|---|---|
| ep/CA | GPa | MPa | Ø |
| 1/0.8 | 3.2 ± 0.1 | 121 ± 14 | 0.066 ± 0.023 |
| E′ at 40 °C | Max Tan(δ) | Crosslinking Density | ||
|---|---|---|---|---|
| Hardener | GPa | °C | Amplitude | mol cm−3 |
| CA | 2.23 | 165 | 0.75 | 3.66 |
| HHPA | 2.59 | 140 | 0.91 | 4.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, Q.; Pourchet, S.; Placet, V.; Plasseraud, L.; Boni, G. New Eco-Friendly Synthesized Thermosets from Isoeugenol-Based Epoxy Resins. Polymers 2020, 12, 229. https://doi.org/10.3390/polym12010229
Ruiz Q, Pourchet S, Placet V, Plasseraud L, Boni G. New Eco-Friendly Synthesized Thermosets from Isoeugenol-Based Epoxy Resins. Polymers. 2020; 12(1):229. https://doi.org/10.3390/polym12010229
Chicago/Turabian StyleRuiz, Quentin, Sylvie Pourchet, Vincent Placet, Laurent Plasseraud, and Gilles Boni. 2020. "New Eco-Friendly Synthesized Thermosets from Isoeugenol-Based Epoxy Resins" Polymers 12, no. 1: 229. https://doi.org/10.3390/polym12010229
APA StyleRuiz, Q., Pourchet, S., Placet, V., Plasseraud, L., & Boni, G. (2020). New Eco-Friendly Synthesized Thermosets from Isoeugenol-Based Epoxy Resins. Polymers, 12(1), 229. https://doi.org/10.3390/polym12010229