Poly(d,l-Lactic acid) Composite Foams Containing Phosphate Glass Particles Produced via Solid-State Foaming Using CO2 for Bone Tissue Engineering Applications
Abstract
:1. Introduction
2. Experimental
2.1. Composite and Foam Fabrication
2.2. Composite and Foam Characterization
2.2.1. Density and Molecular Weight (Mw) of the Polymer Matrix after Composite Processing
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Computed X-ray Micro-Tomography (Micro-CT)
2.2.4. Mechanical Analysis
2.3. Statistical Analysis
3. Results
3.1. PDLLA-PGP Composite Monoliths (Nonporous)
3.1.1. Physical Properties
3.1.2. Morphological Characterization
3.1.3. Mechanical Properties
3.2. PDLLA-PGP Composite Foams
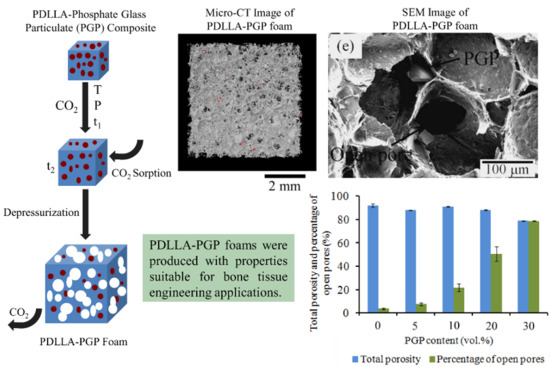
3.2.1. Morphological Characterization
3.2.2. Micro-CT
3.2.3. Mechanical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.T. Biologic biomaterials: Tissue-derived biomaterials (collagen). In Biomaterials: Principles and Applications; Park, J.B., Bronzino, J.D., Eds.; CRC Press: New York, NY, USA, 2002; pp. 118–142. [Google Scholar]
- Hutmacher, D.W.; Garcia, A.J. Scaffold-based bone engineering by using genetically modified cells. Gene 2005, 347, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.P.; Ushida, T.; Tateishi, T. Scaffold design for tissue engineering. Macromol. Biosci. 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomat. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.; Blaker, J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Morphological examination of highly porous polylactic acid/Bioglass® scaffolds produced via nonsolvent induced phase separation. J. Biomed. Mater. Res. B 2017, 105B, 2433–2442. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Lu, H.H. Polymer-ceramic composites for bone tissue engineering. In Bone Engineering; Davies, J.E., Ed.; EM Square Incorporated: Toronto, ON, Canada, 2000; pp. 462–472. [Google Scholar]
- Wang, M. Developing bioactive composite materials for tissue replacement. Biomaterials 2003, 24, 2133–2151. [Google Scholar] [CrossRef]
- Blaker, J.J.; Maquet, V.; Jerome, R.; Boccaccini, A.R.; Nazhat, S.N. Mechanical properties of highly porous PDLLA/Bioglass® composite foams as scaffolds for bone tissue engineering. Acta Biomater. 2005, 1, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Engelberg, I.; Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: A comprehensive study. Biomaterials 1991, 12, 292–304. [Google Scholar] [CrossRef]
- Nazhat, S.N.; Kellomaki, M.; Tormala, P.; Tanner, K.E.; Bonfield, W. Dynamic mechanical characterization of biodegradable composites of hydroxyapatite and polylactides. J. Biomed. Mater. Res. 2001, 58, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, A.M.P.; deWijn, J.R.; vanderMeer, S.A.T.; deGroot, K. Characterization of silane-treated hydroxyapatite powders for use as filler in biodegradable composites. J. Biomed. Mater. Res. 1996, 30, 231–238. [Google Scholar] [CrossRef]
- Patel, A.; Knowles, J.C. Investigation of silica-iron-phosphate glasses for tissue engineering. J. Mater. Sci. Mater. Med. 2006, 17, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.S.; Tan, T.N.; Wang, D.H. Effect of composition on the release kinetics of phosphate controlled release glasses in aqueous medium. J. Control. Release 2004, 96, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Franks, K.; Abrahams, I.; Knowles, J.C. Development of soluble glasses for biomedical use Part I: In vitro solubility measurement. J. Mater. Sci. Mater. Med. 2000, 11, 609–614. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Production of porous polylactic acid monoliths via nonsolvent induced phase separation. Polymer 2014, 55, 6743–6753. [Google Scholar] [CrossRef]
- Gay, S.; Lefebvre, G.; Bonnin, M.; Nottelet, B.; Boury, F.; Gibaud, A.; Calvignac, B. PLA scaffolds production from Thermally Induced Phase Separation: Effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide. J. Supercrit. Fluids 2018, 136, 123–135. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Demarquette, N.R. Ultraporous membranes electrospun from nonsolvent induced phase separated ternary systems. Macromol. Rapid Commun. 2019, 40. [Google Scholar] [CrossRef]
- Griffanti, G.; James-Bhasin, M.; Donelli, I.; Freddi, G.; Nazhat, S.N. Functionalization of silk fibroin through anionic fibroin derived polypeptides. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladapo, B.I.; Zahedi, S.A.; Adeoye, A.O.M. 3D printing of bone scaffolds with hybrid biomaterials. Compos. Part B 2019, 158, 428–436. [Google Scholar] [CrossRef]
- Barry, J.J.; Silva, M.M.; Popov, V.K.; Shakesheff, K.M.; Howdle, S.M. Supercritical carbon dioxide: Putting the fizz into biomaterials. Philos. Trans. R. Soc. A 2006, 364, 249–261. [Google Scholar] [CrossRef]
- Quirk, R.A.; France, R.M.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid technologies and tissue engineering scaffolds. Curr. Opin. Solid State Mater. 2004, 8, 313–321. [Google Scholar] [CrossRef]
- Cooper, A.I. Porous materials and supercritical fluids. Adv. Mater. 2003, 15, 1049–1059. [Google Scholar] [CrossRef]
- Georgiou, G.; Mathieu, L.; Pioletti, D.P.; Bourban, P.E.; Manson, J.A.E.; Knowles, J.C.; Nazhat, S.N. Polylactic acid-phosphate glass composite foams as scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B 2007, 80, 322–331. [Google Scholar] [CrossRef]
- Mooney, D.J.; Baldwin, D.F.; Suh, N.P.; Vacanti, L.P.; Langer, R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 1996, 17, 1417–1422. [Google Scholar] [CrossRef]
- Shah Mohammadi, M.; Chicatun, F.; Stahli, C.; Muja, N.; Burau, M.N.; Nazhat, S.N. Osteoblastic differentiation under controlled bioactive ion release by silica and titania doped sodium-free calcium phosphate-based glass. Colloids Surf. B Biointerfaces 2014, 121, 82–91. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Three-Dimensional Mineralization of Dense Nanofibrillar Collagen-Bioglass Hybrid Scaffolds. Biomacromolecules 2010, 11, 1470–1479. [Google Scholar] [CrossRef]
- Reignier, J.; Gendron, R.; Champagne, M.F. Extrusion foaming of poly (lactic acid) blown with CO2: Toward 100% green material. Cell. Polym. 2007, 26, 83–115. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Oxford Press: Oxford, UK, 1998; pp. 120–168. [Google Scholar]
- Roberst, A.P.; Garboczi, E.J. Elastic moduli of model random three-dimensional closed-cell cellular solids. Acta Mater. 2001, 49, 189–197. [Google Scholar]
- Shah Mohammadi, M.; Ahmed, I.; Marelli, B.; Rudd, C.; Bureau, M.N.; Nazhat, S.N. Modulation of polycaprolactone composite properties through incorporation of mixed phosphate glass formulations. Acta Biomater. 2010, 6, 3157–3168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah Mohammadi, M.; Ahmed, I.; Muja, N.; Rudd, C.D.; Bureau, M.N.; Nazhat, S.N. Effect of phosphate-based glass fibre surface properties on thermally produced poly(lactic acid) matrix composites. J. Mater. Sci. Mater. Med. 2011, 22, 2659–2672. [Google Scholar] [CrossRef] [PubMed]
- Mondrinos, M.J.; Dembzynski, R.; Lu, L.; Byrapogu, V.K.C.; Wootton, D.M.; Lelkes, P.I.; Zhou, J. Porogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering. Biomaterials 2006, 27, 4399–4408. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.C.M.; Si-Hoe, K.M.; Keh, J.E.L.; Teoh, S.H. Correlation of cancellous bone microarchitectural parameters from microCT to CT number and bone mechanical properties. Mater. Sci. Eng. C 2007, 27, 333–339. [Google Scholar] [CrossRef]
- Vitins, V.; Dobelis, M.; Middleton, J.; Limbert, G.; Knets, I. Flexural and creep properties of human jaw compact bone for FEA studies. Comput. Methods Biomech. Biomed. Eng. 2003, 6, 299–303. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biornaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Qaiss, A.; Saidi, H.; Fassi-Fehri, O.; Bousmina, M. Porosity formation by biaxial stretching in polyolefin films filled with calcium carbonate particles. J. Appl. Polym. Sci. 2012, 123, 3425–3436. [Google Scholar] [CrossRef]
- Bureau, M.N. The relationship between morphology and mechanical properties in thermoplastic foams. In Thermoplastic Foam Processing: Principles and Development; Gendron, R., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 235–281. [Google Scholar]
- Torres, F.G.; Nazhat, S.N.; Sheikh, M.D.; Fadzullah, S.H.; Maquet, V.; Boccaccini, A.R. Mechanical properties and bioactivity of porous PLGA/TiO2 nanoparticle-filled composites for tissue engineering scaffolds. Compos. Sci. Technol. 2007, 67, 1139–1147. [Google Scholar] [CrossRef]
- Salerno, A.; Zeppetelli, S.; Di Maio, E.; Iannace, S.; Netti, P.A. Design of Bimodal PCL and PCL-HA Nanocomposite Scaffolds by Two Step Depressurization During Solid-state Supercritical CO2 Foaming. Macromol. Rapid Commun. 2011, 32, 1150–1156. [Google Scholar] [CrossRef]
- Salerno, A.; Zeppetelli, S.; Di Maio, E.; Iannace, S.; Netti, P.A. Architecture and properties of bi-modal porous scaffolds for bone regeneration prepared via supercritical CO2 foaming and porogen leaching combined process. J. Supercrit. Fluids 2012, 67, 114–122. [Google Scholar] [CrossRef]
- Mathieu, L.M.; Montjovent, M.-O.; Bourban, P.-E.; Pioletti, D.P.; Månson, J.-A.E. Bioresorbable composites prepared by supercritical fluid foaming. J. Biomed. Mater. Res. 2005, 75, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Ginebra, M.P.; Planell, J.A.; Zeppetelli, S.; Ambrosio, L. Development and cell response of a new biodegradable composite scaffold for guided bone regeneration. J. Mater. Sci. Mater. Med. 2004, 15, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Knowles, J.C.; Day, R.M. Novel fabrication techniques to produce microspheres by thermally induced phase separation for tissue engineering and drug delivery. Acta Biomater. 2008, 4, 264–272. [Google Scholar] [CrossRef] [PubMed]






| Material Code | PGP (vol.%) | PGP Content Experimental (wt.%) | Density (g.cm−3) | PDLLA Molecular Weight (Mw: Daltons) | Relative Mw |
|---|---|---|---|---|---|
| PDLLA | - | - | 1.26 | 51,594 ± 131 | 1.000 |
| PDLLA-5PGP | 5 | 9.8 ± 0.65 | 1.27 ± 0.017 | 51,133 ± 149 | 0.991 |
| PDLLA-10PGP | 10 | 16.6 ± 0.09 | 1.35 ± 0.016 | 50,954 ± 91 | 0.988 |
| PDLLA-20PGP | 20 | 32.9 ± 0.08 | 1.51 ± 0.017 | 49,936 ± 13 | 0.968 |
| PDLLA-30PGP | 30 | 45.9 ± 0.14 | 1.63 ± 0.02 | 51,445 ± 211 | 0.997 |
| Material Code | Pore Size (µm) | Total Porosity (vol.%) | Percentage of Open Pores |
|---|---|---|---|
| PDLLA | 920 ± 640 | 92 ± 1.59 | 3.7 ± 0.65 |
| PDLLA-5PGP | 530 ± 230 | 87.9 ± 0.44 | 7.7 ± 1.17 |
| PDLLA-10PGP | 270 ± 210 | 91 ± 0.51 | 22 ± 3.47 |
| PDLLA-20PGP | 230 ± 140 | 88 ± 0.48 | 50.4 ± 6.24 |
| PDLLA-30PGP | 190 ± 130 | 78.8 ± 0.35 | 78.6 ± 0.35 |
| Material Code | E (MPa) | E0 (GPa) | n |
|---|---|---|---|
| PDLLA | 2.22 ± 0.24 | 3.36 ± 0.22 | 2.68 |
| PDLLA-5PGP | 2.79 ± 0.40 | 3.62 ± 0.28 | 2.49 |
| PDLLA-10PGP | 4.89 ± 0.14 | 4.05 ± 0.33 | 2.31 |
| PDLLA-20PGP | 5.65 ± 0.22 | 4.91 ± 0.31 | 2.67 |
| PDLLA-30PGP | 7.27 ± 0.65 | 6.19 ± 0.45 | 3.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah Mohammadi, M.; Rezabeigi, E.; Bertram, J.; Marelli, B.; Gendron, R.; Nazhat, S.N.; Bureau, M.N. Poly(d,l-Lactic acid) Composite Foams Containing Phosphate Glass Particles Produced via Solid-State Foaming Using CO2 for Bone Tissue Engineering Applications. Polymers 2020, 12, 231. https://doi.org/10.3390/polym12010231
Shah Mohammadi M, Rezabeigi E, Bertram J, Marelli B, Gendron R, Nazhat SN, Bureau MN. Poly(d,l-Lactic acid) Composite Foams Containing Phosphate Glass Particles Produced via Solid-State Foaming Using CO2 for Bone Tissue Engineering Applications. Polymers. 2020; 12(1):231. https://doi.org/10.3390/polym12010231
Chicago/Turabian StyleShah Mohammadi, Maziar, Ehsan Rezabeigi, Jason Bertram, Benedetto Marelli, Richard Gendron, Showan N. Nazhat, and Martin N. Bureau. 2020. "Poly(d,l-Lactic acid) Composite Foams Containing Phosphate Glass Particles Produced via Solid-State Foaming Using CO2 for Bone Tissue Engineering Applications" Polymers 12, no. 1: 231. https://doi.org/10.3390/polym12010231
APA StyleShah Mohammadi, M., Rezabeigi, E., Bertram, J., Marelli, B., Gendron, R., Nazhat, S. N., & Bureau, M. N. (2020). Poly(d,l-Lactic acid) Composite Foams Containing Phosphate Glass Particles Produced via Solid-State Foaming Using CO2 for Bone Tissue Engineering Applications. Polymers, 12(1), 231. https://doi.org/10.3390/polym12010231