Copolymerization of Propylene with Higher α-Olefins by a Pyridylamidohafnium Catalyst: An Effective Approach to Polypropylene-Based Elastomer
Abstract
:1. Introduction
2. Experimental Section
2.1. General Procedure and Materials
2.2. General Procedure for Copolymerization
2.3. Preparation of ESI-MS Sample
3. Characterization
4. Results and Discussions
4.1. The Copolymerization of Propylene with Higher α-Olefins by Dimethyl (pyridyl-amido)Hafnium Catalyst
4.2. Microstructure and Thermal Behavior
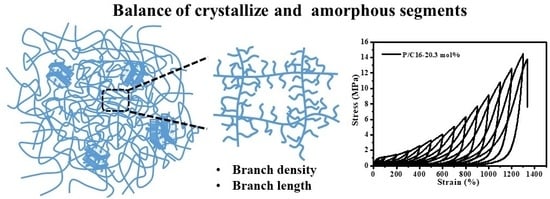
4.3. Mechanical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific olefin polymerization with chiral metallocene catalysts. Angew. Chem. Int. Ed. 1995, 34, 1143–1170. [Google Scholar] [CrossRef] [Green Version]
- Quijada, R.; Galland, G.B.; Mauler, R.S. The influence of the comonomer in the copolymerization of ethylene with α-olefins using C2H4[ind]2ZrCl2/methylaluminoxane as catalyst system. Macromol. Chem. Phys. 1996, 197, 3091–3098. [Google Scholar] [CrossRef]
- Boaen, N.K.; Hillmyer, M.A. Post-polymerization functionalization of polyolefins. Chem. Soc. Rev. 2005, 34, 267–275. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Kirillov, E.; Carpentier, J.F. Group 3 and 4 single-site catalysts for stereospecific polymerization of styrene. Coordin. Chem. Rev. 2008, 252, 2115–2136. [Google Scholar] [CrossRef]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The influence of ziegler-natta and metallocene catalysts on polyolefin structure, properties, and processing ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef]
- Kravchenko, R.; Masood, A.; Waymouth, R.M.; Myers, C.L. Strategies for synthesis of elastomeric polypropylene: Fluxional metallocenes with c1-symmetry. J. Am. Chem. Soc. 1998, 120, 2039–2046. [Google Scholar] [CrossRef]
- Maciejewski Petoff, J.L.; Agoston, T.; Lal, T.K.; Waymouth, R.M. Elastomeric polypropylene from unbridged 2-arylindenyl zirconocenes: Modeling polymerization behavior using ansa-metallocene analogues. J. Am. Chem. Soc. 1998, 120, 11316–11322. [Google Scholar] [CrossRef]
- Saito, J.; Suzuki, Y.; Makio, H.; Tanaka, H.; Onda, M.; Fujita, T. Polymerization of higher α-olefins with a bis(phenoxyimine)Ti complex/i-Bu3Al/Ph3CB(C6F5)4: Formation of stereo- and regioirregular high molecular weight polymers with high efficiency. Macromolecules 2006, 39, 4023–4031. [Google Scholar] [CrossRef]
- Domski, G.J.; Lobkovsky, E.B.; Coates, G.W. Polymerization of α-olefins with pyridylamidohafnium catalysts: Living behavior and unexpected isoselectivity from a Cs-symmetric catalyst precursor. Macromolecules 2007, 40, 3510–3513. [Google Scholar] [CrossRef]
- Wang, H.P.; Khariwala, D.U.; Cheung, W.; Chum, S.P.; Hiltner, A.; Baer, E. Characterization of some new olefinic block copolymers. Macromolecules 2007, 40, 2852–2862. [Google Scholar] [CrossRef]
- Gao, R.; Sun, W.H.; Redshaw, C. Nickel complex pre-catalysts in ethylene polymerization: New approaches to elastomeric materials. Catal. Sci. Technol. 2013, 3, 1172–1179. [Google Scholar] [CrossRef]
- Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T.; Arriola, D.J.; Carnahan, E.M. Chain shuttling catalysis and olefin block copolymers. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 699–737. [Google Scholar]
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies-history and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Ostoja Starzewski, A.; Steinhauser, N.; Xin, B.S. Decisive progress in metallocene-catalyzed elastomer synthesis. Macromolecules 2008, 41, 4095–4101. [Google Scholar] [CrossRef]
- Zhang, K.L.; Liu, P.W.; Wang, W.J.; Li, B.G.; Liu, W.F.; Zhu, S.P. Preparation of comb-shaped polyolefin elastomer having ethylene/1-octene copolymer backbone and long chain polyethylene branches via a tandem metallocene catalyst system. Macromolecules 2018, 51, 8790–8799. [Google Scholar] [CrossRef]
- Burns, A.B.; Register, R.A. Thermoplastic elastomers via combined crystallization and vitrification from homogeneous melts. Macromolecules 2015, 49, 269–279. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.T.; Fu, L.L.; Jiang, Z.Y.; Men, Y.F. Crystallization, recrystallization, and melting lines in syndiotactic polypropylene crystallized from quiescent melt and semicrystalline state due to stress-induced localized melting and recrystallization. J. Phys. Chem. B 2014, 118, 13019–13023. [Google Scholar] [CrossRef]
- Song, X.Y.; Cao, L.X.; Tanaka, R.; Shiono, T.; Cai, Z.G. Optically transparent functional polyolefin elastomer with excellent mechanical and thermal properties. ACS Macro Lett. 2019, 8, 299–303. [Google Scholar] [CrossRef]
- Müller, G.; Rieger, B. Propene based thermoplastic elastomers by early and late transition metal catalysis. Prog. Polym. Sci. 2002, 27, 815–851. [Google Scholar] [CrossRef]
- Hotta, A.; Cochran, E.; Ruokolainen, J.; Khanna, V.; Fredrickson, G.H.; Kramer, E.J.; Shin, Y.W.; Shimizu, F.; Cherian, A.E.; Hustad, P.D.; et al. Semicrystalline thermoplastic elastomeric polyolefins: Advances through catalyst development and macromolecular design. Proc. Natl. Acad. Sci. USA 2006, 103, 15327–15332. [Google Scholar] [CrossRef] [Green Version]
- Ohtaki, H.; Deplace, F.; Vo, G.D.; LaPointe, A.M.; Shimizu, F.; Sugano, T.; Kramer, E.J.; Fredrickson, G.H.; Coates, G.W. Allyl-terminated polypropylene macromonomers: A route to polyolefin elastomers with excellent elastic behavior. Macromolecules 2015, 48, 7489–7494. [Google Scholar] [CrossRef]
- Wu, Q.; Su, Q.; Ye, L.; Li, G.; Mu, Y. Propylene polymerization to high molecular weight atactic polypropylene and copolymerization with 1-hexene using monocyclopentadienyl titanium catalysts. Dalton Trans. 2010, 39, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Mehtarani, R.; Fu, Z.; Fan, Z.; Tu, S.; Feng, L.F. Synthesis of polypropylene/poly(ethylene-co-propylene) in-reactor alloys by periodic switching polymerization process-effects of gas phase polymerization time on polymer properties. Ind. Eng. Chem. Res. 2013, 52, 13556–13563. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, J.; Sita, L.R. Living coordinative chain-transfer polymerization and copolymerization of ethene, α-olefins, and α,ω-nonconjugated dienes using dialkylzinc as “surrogate” chain-growth sites. Macromolecules 2008, 41, 7829–7833. [Google Scholar] [CrossRef]
- Harney, M.B.; Zhang, Y.; Sita, L.R. Discrete, multiblock isotactic–atactic stereoblock polypropene microstructures of differing block architectures through programmable stereomodulated living ziegler–natta polymerization. Angew. Chem. Int. Ed. 2006, 45, 2400–2404. [Google Scholar] [CrossRef]
- Machat, M.R.; Lanzinger, D.; Drees, M.; Altmann, P.J.; Herdtweck, E.; Rieger, B. High-melting, elastic polypropylene: A one-pot, one-catalyst strategy toward propylene-based thermoplastic elastomers. Macromolecules 2018, 51, 914–929. [Google Scholar] [CrossRef]
- Kukral, J.; Lehmus, P.; Feifel, T.; Troll, C.; Rieger, B. Dual-side ansa-zirconocene dichlorides for high molecular weight isotactic polypropene elastomers. Organometallics 2000, 19, 3767–3775. [Google Scholar] [CrossRef]
- Rieger, B.; Troll, C.; Preuschen, J. Ultrahigh molecular weight polypropene elastomers by high activity "dual-side" hafnocene catalysts. Macromolecules 2002, 35, 5742–5743. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Friederichs, N.; Ronca, S.; Togrou, M. The first molecularly characterized isotactic polypropylene-block-polyethylene obtained via “quasi-living” insertion polymerization. Macromolecules 2003, 36, 3806–3808. [Google Scholar] [CrossRef]
- Mahanthappa, M.K.; Hillmyer, M.A.; Bates, F.S. Mechanical consequences of molecular composition on failure in polyolefin composites containing glassy, elastomeric, and semicrystalline components. Macromolecules 2008, 41, 1341–1351. [Google Scholar] [CrossRef]
- Yuan, X.; Matsuyama, Y.; Chung, T.C.M. Synthesis of functionalized isotactic polypropylene dielectrics for electric energy storage applications. Macromolecules 2010, 43, 4011–4015. [Google Scholar] [CrossRef]
- He, Z.; Niu, H.; Zheng, N.; Liu, S.; Li, Y. Poly(ethylene-co-propylene)/poly(ethylene glycol) elastomeric hydrogels with thermoreversibly cross-linked networks. Polym. Chem. 2019, 10, 4789–4800. [Google Scholar] [CrossRef]
- Shi, X.C.; Tang, X.Y.; Li, Y.S. Random copolymers of propylene with 1,5-hexadiene containing only cyclopentane units in main chain and tailoring structure and mechanical properties of the copolymers. Polymer 2011, 52, 3053–3058. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.X.; Cui, J.; Long, Y.Y.; Li, Y.G.; Yuan, X.Y.; Li, Y.S. Cyclopolymerization of Si-containing α,ω-Diolefins by a pyridylamidohafnium catalyst with high cyclization selectivity and stereoselectivity. Macromolecules 2014, 47, 6627–6634. [Google Scholar] [CrossRef]
- Rose, J.M.; Cherian, A.E.; Lee, J.H.; Archer, L.A.; Coates, G.W.; Fetters, L.J. Rheological behavior of chain-straightened poly(α-olefin)s. Macromolecules 2007, 40, 6807–6813. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Tsou, A.H.; Hagadorn, J.R.; Throckmorton, J.A. Highly entangled α-olefin molecular bottlebrushes: Melt structure, linear rheology, and interchain friction mechanism. Macromolecules 2018, 51, 6958–6966. [Google Scholar] [CrossRef]
- Numura, K.; Itagaki, K.; Fujiki, M. Efficient incorporation of 2-methyl-1-pentene in copolymerization of ethylene with 2-methyl-1-pentene catalyzed by nonbridged half-titanocenes. Macromolecules 2005, 38, 2053–2055. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Wang, Z.; Sheiko, S.S.; Dobrynin, A.V. Combs and bottlebrushes in a melt. Macromolecules 2017, 50, 3430–3437. [Google Scholar] [CrossRef]
- O’Connor, K.S.; Watts, A.; Vaidya, T.; LaPointe, A.M.; Hillmyer, M.A.; Coates, G.W. Controlled chain walking for the synthesis of thermoplastic polyolefin elastomers: Synthesis, structure, and properties. Macromolecules 2016, 49, 6743–6751. [Google Scholar] [CrossRef]
- Na, Y.; Dai, S.; Chen, C. Direct synthesis of polar-functionalized linear low-density polyethylene (LLDPE) and low-density polyethylene (LDPE). Macromolecules 2018, 51, 4040–4048. [Google Scholar] [CrossRef]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic production of olefin block copolymers via chain shuttling polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef]
- Cueny, E.S.; Johnson, H.C.; Anding, B.J.; Landis, C.R. Mechanistic studies of hafnium-pyridyl amido-catalyzed 1-octene polymerization and chain transfer using quench-labeling methods. J. Am. Chem. Soc. 2017, 139, 11903–11912. [Google Scholar] [CrossRef] [PubMed]
- Graef, S.M.; Wahner, U.M.; Van Reenen, A.J.; Brüll, R.; Sanderson, R.D.; Pasch, H. Copolymerization of propylene with higher α-olefins in the presence of the syndiospecific catalyst i-Pr(Cp)(9-Flu)ZrCl2/MAO. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 128–140. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Miyata, H.; Nitta, K.H. Compatibility of binary blends of polypropylene with ethylene-α-olefin copolymer. J. Polym. Sci. Part A Polym. Chem. 1996, 62, 87–97. [Google Scholar] [CrossRef]
- Jian, Z.; Mecking, S. Short-Chain branched polar-functionalized linear polyethylene via "tandem catalysis". Macromolecules 2016, 49, 4057–4066. [Google Scholar] [CrossRef]
- Karbach, F.F.; Macko, T.; Duchateau, R. Preparation of ethylene/1-hexene copolymers from ethylene using a fully silica-supported tandem catalyst system. Macromolecules 2016, 49, 1229–1241. [Google Scholar] [CrossRef]
- Lizuka, Y.; Sugiyama, J.-I.; Hagihara, H. Unexpected mechanical properties of functionalized polypropylene: Tensile test, charpy impact tensile test, DSC, and WAXD analysis of poly(5-hexen-1-ol-co-propylene). Macromolecules 2009, 42, 2321–2323. [Google Scholar]
- Schwerdtfeger, E.D.; Price, C.J.; Chai, J.; Miller, S.A. Tandem catalyst system for linear low-density polyethylene with short and long branching. Macromolecules 2010, 43, 4838–4842. [Google Scholar] [CrossRef]
- Arnold, M.; Henschke, O.; Knorr, J. Copolymerization of propene and higher α-olefins with the metallocene catalyst Et[Ind]2 HfC12)/methylaluminoxane. Macromol. Chem. Phys. 1996, 197, 563–573. [Google Scholar] [CrossRef]
- Poon, B.; Rogunova, M.; Hiltner, A.; Baer, E.; Chum, S.P.; Galeski, A.; Piorkowska, E. Structure and properties of homogeneous copolymers of propylene and 1-hexene. Macromolecules 2005, 38, 1232–1243. [Google Scholar] [CrossRef]
- Alt, H.G.; Köppl, A. Effect of the nature of metallocene complexes of group IV metals on their performance in catalytic ethylene and propylene polymerization. Chem. Rev. 2000, 100, 1205–1222. [Google Scholar] [CrossRef]
- Angermund, K.; Fink, G.; Jensen, V.R.; Kleinschmidt, R. Toward quantitative prediction of stereospecificity of metallocene-based catalysts for α-olefin polymerization. Chem. Rev. 2000, 100, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Piel, C.; Stadler, F.J.; Kaschta, J.; Rulhoff, S.; Münstedt, H.; Kaminsky, W. Structure-property relationships of linear and long-chain branched metallocene high-density polyethylenes characterized by shear rheology and sec-malls. Macromol. Chem. Phys. 2006, 207, 26–38. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, F.; Ma, Z.; Pan, L.; Li, Y.S. Structure and property of comb-like polyoelfins derived from highly stereospecific homo-polymerization of higher α-olefins. Polymer 2019. Submmited. [Google Scholar]
- Kaminsky, W.; Piel, C.; Scharlach, K. Polymerization of ethene and longer chained olefins by metallocene catalysis. Macromol. Symp. 2005, 226, 25–34. [Google Scholar] [CrossRef]
- Rulhoff, S.; Kaminsky, W. Synthesis and characterization of defined branched poly(propylene)s with different microstructures by copolymerization of propylene and linear ethylene oligomers (Cn = 26 − 28) with metallocenes/MAO catalysts. Macromol. Chem. Phys. 2006, 207, 1450–1460. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Mechanism of activation of a hafnium pyridyl−amide olefin polymerization catalyst: Ligand modification by monomer. J. Am. Chem. Soc. 2007, 129, 7831–7840. [Google Scholar] [CrossRef]
- Zuccaccia, C.; Macchioni, A.; Busico, V.; Cipullo, R.; Talarico, G.; Alfano, F.; Boone, H.W.; Frazier, K.A.; Hustad, P.D.; Stevens, J.C.; et al. Intra- and intermolecular NMR studies on the activation of arylcyclometallated hafnium pyridyl-amido olefin polymerization precatalysts. J. Am. Chem. Soc. 2008, 130, 10354–10368. [Google Scholar] [CrossRef]
- Zuccaccia, C.; Busico, V.; Cipullo, R.; Talarico, G.; Froese, R.D.J.; Vosejpka, P.C.; Hustad, P.D.; Macchioni, A. On the first insertion of α-olefins in hafnium pyridyl-amido polymerization catalysts. Organometallics 2009, 28, 5445–5458. [Google Scholar] [CrossRef]
- Miller, S.A.; Bercaw, J.E. Mechanism of isotactic polypropylene formation with c1-symmetric metallocene catalysts. Organometallics 2006, 25, 3576–3592. [Google Scholar] [CrossRef]
- Zhang, X.B.; Li, Z.S.; Lu, Z.Y.; Sun, C.C. Roles of branch content and branch length in copolyethylene crystallization: Molecular dynamics simulations. Macromolecules 2002, 35, 106–111. [Google Scholar] [CrossRef]
- Schrauwen, B.A.G.; Janssen, R.P.M.; Govaert, L.E.; Meijer, H.E.H. Intrinsic deformation behavior of semicrystalline polymers. Macromolecules 2004, 37, 6069–6078. [Google Scholar] [CrossRef] [Green Version]
- Men, Y.; Rieger, J.; Strobl, G. Role of the entangled amorphous network in tensile deformation of semicrystalline polymers. Phys. Rev. Lett. 2003, 91, 095502. [Google Scholar] [CrossRef] [PubMed]
- Saalwächter, K.; Herrero, B.; López-Manchado, M.A. Chain order and cross-link density of elastomers as investigated by proton multiple-quantum NMR. Macromolecules 2005, 38, 9650–9660. [Google Scholar] [CrossRef]
- Hiss, R.; Hobeika, S.; Lynn, C.; Strobl, G. Network stretching, slip processes, and fragmentation of crystallites during uniaxial drawing of polyethylene and related copolymers. A comparative study. Macromolecules 1999, 32, 4390–4403. [Google Scholar] [CrossRef]
- Orza, R.A.; Magusin, P.C.M.M.; Litvinov, V.M.; van Duin, M.; Michels, M.A.J. Solid-state 1H NMR study on chemical cross-links, chain entanglements, and network heterogeneity in peroxide-cured EPDM rubbers. Macromolecules 2007, 40, 8999–9008. [Google Scholar] [CrossRef]








| Entry | Comonomer (mmol) | Incorp b (mol.%) | Act c | Mwd (kg/mol) | PDI d | Tme (°C) | Xcf (%) |
|---|---|---|---|---|---|---|---|
| 1 | 3.0 | 295 | 2.0 | 160 | 54 | ||
| 2 | 1-octene(1.50) | 3.1 | 3.6 | 335 | 1.6 | 117 | 26 |
| 3 | 1-octene(3.00) | 6.0 | 4.1 | 417 | 1.6 | 92 | 19 |
| 4 | 1-octene(4.50) | 12.2 | 4.7 | 467 | 1.4 | 79 | 14 |
| 5 | 1-octene(6.00) | 19.6 | 5.0 | 519 | 1.4 | 46 | 9 |
| 6 | 1-dodecene(1.50) | 3.0 | 4.0 | 586 | 1.4 | 118 | 28 |
| 7 | 1-dodecene(3.00) | 5.9 | 4.5 | 678 | 1.5 | 101 | 18 |
| 8 | 1-dodecene(4.50) | 12.1 | 4.7 | 651 | 1.4 | 46 | 11 |
| 9 | 1-dodecene(6.00) | 20.5 | 5.1 | 960 | 1.4 | 33 | 9 |
| 10 | 1-hexadecene(1.50) | 3.1 | 4.2 | 603 | 1.5 | 115 | 24 |
| 11 | 1-hexadecene(3.00) | 6.2 | 4.7 | 696 | 1.4 | 95 | 18 |
| 12 | 1-hexadecene(4.50) | 12.2 | 4.9 | 1251 | 1.6 | 46 | 11 |
| 13 | 1-hexadecene(6.00) | 20.3 | 5.2 | 1416 | 1.5 | 34 | 10 |
| 14 | 1-eicosene(1.50) | 3.1 | 4.6 | 689 | 1.4 | 120 | 23 |
| 15 | 1-eicosene(3.00) | 6.0 | 5.1 | 1482 | 1.5 | 115 | 16 |
| 16 | 1-eicosene(4.50) | 12.2 | 5.3 | 1505 | 1.4 | 114 | 10 |
| 17 | 1-eicosene(6.00) | 18.2 | 5.7 | 1678 | 1.4 | 80 | 8 |
| Sample | E (MPa) | σy (Mpa) | εy (%) | σb (Mpa) | εb (%) |
|---|---|---|---|---|---|
| iPP | 600 ± 15 | 40.5 ± 1 | 13 ± 0.5 | ||
| P/C8-12.19 | 25.3 ± 2.2 | 3.7 ± 0.1 | 35 ± 1 | 38 ± 3 | 950 ± 20 |
| P/C8-19.60 | 13 ± 0.1 | 35 ± 2 | 1000 ± 40 | ||
| P/C12-12.09 | 4.0 ± 0.2 | 3.2 ± 0.1 | 34 ± 1 | 33 ± 3 | 1100 ± 40 |
| P/C12-20.50 | 2.2 ± 0.2 | 17 ± 1 | 1150 ± 20 | ||
| P/C16-12.22 | 3.8 ± 0.1 | 1.6 ± 0.1 | 32 ± 1 | 28 ± 3 | 1100 ± 20 |
| P/C16-20.30 | 3.2 ± 0.4 | 18 ± 2 | 1100 ± 30 | ||
| P/C20-3.06 | 157 ± 5 | 12.9 ± 0.3 | 21 ± 2 | 30 ± 2 | 1100 ± 20 |
| P/C20-5.96 | 45 ± 2 | 5.3 ± 0.1 | 24 ± 1 | 26 ± 1 | 1050 ± 30 |
| P/C20-12.24 | 2.7 ± 0.1 | 2.9 ± 0.1 | 32 ± 1 | 25 ± 3 | 1140 ± 50 |
| P/C20-18.24 | 1.8 ± 0.1 | 15 ± 1 | 1200 ± 30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Wang, X.; Ma, Z.; Wang, B.; Pan, L.; Li, Y. Copolymerization of Propylene with Higher α-Olefins by a Pyridylamidohafnium Catalyst: An Effective Approach to Polypropylene-Based Elastomer. Polymers 2020, 12, 89. https://doi.org/10.3390/polym12010089
Yang F, Wang X, Ma Z, Wang B, Pan L, Li Y. Copolymerization of Propylene with Higher α-Olefins by a Pyridylamidohafnium Catalyst: An Effective Approach to Polypropylene-Based Elastomer. Polymers. 2020; 12(1):89. https://doi.org/10.3390/polym12010089
Chicago/Turabian StyleYang, Fei, Xiaoyan Wang, Zhe Ma, Bin Wang, Li Pan, and Yuesheng Li. 2020. "Copolymerization of Propylene with Higher α-Olefins by a Pyridylamidohafnium Catalyst: An Effective Approach to Polypropylene-Based Elastomer" Polymers 12, no. 1: 89. https://doi.org/10.3390/polym12010089
APA StyleYang, F., Wang, X., Ma, Z., Wang, B., Pan, L., & Li, Y. (2020). Copolymerization of Propylene with Higher α-Olefins by a Pyridylamidohafnium Catalyst: An Effective Approach to Polypropylene-Based Elastomer. Polymers, 12(1), 89. https://doi.org/10.3390/polym12010089