The Toxicological Testing and Thermal Decomposition of Drive and Transport Belts Made of Thermoplastic Multilayer Polymer Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. FT-IR Spectroscopy
2.2.2. Density and Shore Hardness
2.2.3. Scanning Electron Microscopy (SEM) and Optical Light Microscopy (PLM)
2.2.4. Thermogravimetric Analysis
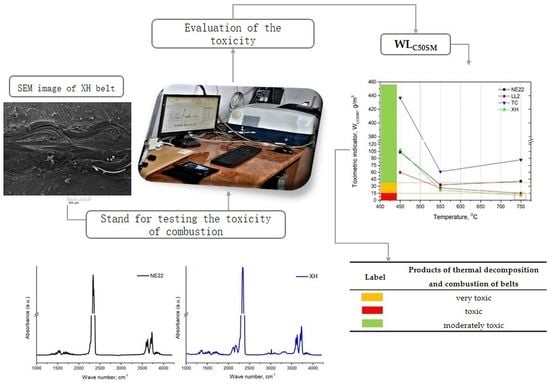
2.3. The Measurement Method of the Toxicity Products of Thermal Decomposition and Combustion
- −
- toxicometric indicator is a mass of a given material whose decomposition or combustion under test conditions produces toxic concentration limits for a given product of thermal decomposition, described by Equation (2):where is an indicator of limit concentration of products of thermal decomposition and combustion, and E is a specific emission of toxic products of thermal decomposition and combustion (g g−1).
- −
- toxicometric indicator is a resultant of indicator for individual products of thermal decomposition and combustion for a given temperature determined according to Equation (3):where n is the number of samples, and is the concentration causing the death of 50% of the population at 30 min exposure, the values of which are indicated in Table 2.
- −
- toxicometric indicator () is an arithmetic average of indicators from individual temperatures (450, 550, and 750 °C), described by Equation (4):
3. Results and Discussions
3.1. FT-IR Scpectoscopy Chemical Composition Analysis
3.2. Values of Toxicometric Indicators Analysis
3.3. Assessment of Products of Thermal Decomposition and Combustion
3.4. The Thermal Decomposition Kinetics of Belts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domek, G.; Kołodziej, A.; Wilczyński, M.; Krawiec, P. The problem of cooperation of a flat belts with elements of mechatronic systems. In Proceedings of the 55th International Conference on Experimental Stress Analysis, Novy Smokovec, Slovakia, 30 May–1 June 2017; pp. 706–711. [Google Scholar]
- Fedorko, G.; Molnár, V.; Živčák, J.; Dovica, M.; Husáková, N. Failure analysis of textile rubber conveyor belt damaged by dynamic wear. Eng. Fail. Anal. 2013, 28, 103–114. [Google Scholar] [CrossRef]
- Fedorko, G.; Molnar, V.; Marasova, D.; Grincova, A.; Dovica, M.; Zivcak, J.; Toth, T.; Husakova, N. Failure analysis of belt conveyor damage caused by the falling material. Part I: Experimental measurements and regression models. Eng. Fail. Anal. 2014, 36, 30–38. [Google Scholar] [CrossRef]
- Krawiec, P.; Domek, G.; Adamiec, J.; Waluś, K.J.; Warguła, Ł. The proposal of estimation method of mating between pulleys and cogbelt. In Proceedings of the 55th International Conference on Experimental Stress Analysis, Novy Smokovec, Slovakia, 30 May–1 June 2017; pp. 740–747. [Google Scholar]
- Domek, G.; Krawiec, P.; Wilczynski, M. Timing belt in power transmission and conveying system. In MATEC Web of Conferences, Proceedings of the Machine Modelling and Simulations, SklenéTeplice, Slovak Republic, 5–8 September 2017; EDP Sciences: Lez Ili, France, 2018; Volume 157. [Google Scholar]
- Czarnecka-Komorowska, D.; Mencel, K. Effect of [3-(2-aminoethyl) amino] propyl-heptaisobutyl-polysilsesquioxane nanoparticles on thermal stability and color of polyoxymethylene and polyamide 6. Przem. Chem. 2014, 93, 1997–2000. [Google Scholar]
- Talaśka, K.; Wojtkowiak, D. Modelling mechanical properties of the multilayer composite materials with the polyamide core. In MATEC Web of Conferences, Proceedings of the Machine Modelling and Simulations, Sklené Teplice, Slovak Republic, 5–8 September 2017; EDP Sciences: Lez Ili, France, 2018; Volume 157. [Google Scholar]
- Ondrušová, D.; Labaj, I.; Vršková, J.; Pajtášová, M.; Zvoláneková Mezencevová, V. Application of alternative additives in the polymer composite systems used in automotive industry. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Volume 776, 24th Slovak-Polish International Scientific Conference on Machine Modelling and Simulations—MMS 2019, Liptovský Ján, Slovakia, 3–6 September 2019; IOP Institute of Physics: Bristol, UK, 2020; pp. 1–9. [Google Scholar]
- Kohutiar, M.; Pajtášová, M.; Janík, R.; Papučová, I.; Pagáčová, J.; Pecušová, B.; Labaj, I. Study of selected thermoplastics using dynamic mechanical analysis. In Proceedings of the MATEC Web of Conferences, SklenéTeplice, Slovak Republic, 5–8 September 2017; EDP Sciences: Lez Ili, France, 2018; Volume 157. [Google Scholar]
- Wojtkowiak, D.; Talaśka, K.; Malujda, I.; Domek, G. Analysis of the influence of the cutting edge geometry on parameters of the perforation process for conveyor and transmission belts. In MATEC Web of Conferences, Proceedings of the Machine Modelling and Simulations, Sklené Teplice, Slovak Republic, 5–8 September 2017; EDP Sciences: Lez Ili, France, 2018; Volume 157. [Google Scholar]
- Fedorko, G.; Molnar, V.; Dovica, M.; Toth, T.; Kopas, M. Analysis of pipe conveyor belt damaged by thermal wear. Eng. Fail. Anal. 2014, 45, 41–48. [Google Scholar] [CrossRef]
- Krawiec, P.; Warguła, Ł.; Dziechciarz, A.; Małozięć, D.; Ondrušová, D. Evaluation of chemical compound emissions during thermal decomposition and combustion of V-belts. Przemysł Chem. 2020, 99, 92–98. [Google Scholar]
- Krawiec, P.; Waluś, K.J.; Warguła, Ł.; Adamiec, J. Wear evaluation of elements of V-belt transmission with the application of optical microscope. In MATEC Web of Conferences, Proceedings of the Machine Modelling and Simulations, Sklené Teplice, Slovak Republic, 5–8 September 2017; EDP Sciences: Lez Ili, France, 2018; Volume 157. [Google Scholar]
- Krawiec, P.; Warguła, Ł.; Waluś, K.J.; Adamiec, J. Wear evaluation study of the multiple grooved pulleys with optical method. In MATEC Web of Conferences, Proceedings of the XXIII Polish-Slovak Scientific Conference on Machine Modelling and Simulations, Rydzyna, Poland, 4–7 September 2019; EDP Sciences: Lez Ili, France, 2019; Volume 254. [Google Scholar]
- Andrejiova, M.; Grincova, A.; Marasova, D. Measurement and simulation of impact wear damage to industrial conveyor belts. Wear 2016, 368, 400–407. [Google Scholar] [CrossRef]
- Warguła, Ł.; Kaczmarzyk, P.; Dziechciarz, A. The assessment of fire risk of non-road mobile wood chopping machines. J. Res. Appl. Agric. Eng. 2019, 64, 58–64. [Google Scholar]
- Kaczmarzyk, P.; Małozięć, D.; Warguła, Ł. Research on electrical wiring used in the construction of working machines and vehicles in the aspect of fire protection. J. Mech. Transp. Eng. 2018, 70, 13–24. [Google Scholar]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Zhang, C.; Dong, J.; Chen, X.; Qian, Y.; Li, S. Combustion behavior and thermal pyrolysis kinetics of flame-retardant epoxy composites based on organic–inorganic intumescent flame retardant. J. Therm. Anal. Calorim. 2015, 119, 1759–1767. [Google Scholar] [CrossRef]
- Solorzano, J.A.P.; Moinuddin, K.A.M.; Tretsiakova-McNally, S.; Joseph, P.A. Study of the Thermal Degradation and Combustion Characteristics of Some Materials Commonly Used in the Construction Sector. Polymers 2019, 11, 1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barczewski, M.; Kurańska, M.; Sałasińska, K.; Michałowski, S.; Prociak, A.; Uram, K.; Lewandowski, K. Rigid polyurethane foams modified with thermoset polyester-glass fiber composite waste. Polym. Test. 2020, 81, 106190. [Google Scholar] [CrossRef]
- Pielichowski, K.; Leszczyńska, A. TG-FTIR study of the thermal degradation of polyoxymethylene (POM)/thermoplastic polyurethane (TPU) blends. J. Therm. Anal. Calorim. 2004, 78, 631–637. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers 2019, 11, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Luo, Y.; Guo, X.; Chen, L.; Xu, T.; Jia, D. Structure and Flame-Retardant Actions of Rigid Polyurethane Foams with Expandable Graphite. Polymers 2019, 11, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salasinska, K.; Barczewski, M.; Borucka, M.; Górny, R.L.; Kozikowski, P.; Celiński, M.; Gajek, A. Thermal Stability, Fire and Smoke Behaviour of Epoxy Composites Modified with Plant Waste Fillers. Polymers 2019, 11, 1234. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Teng, H.; Zhang, X.; Zhang, J.; Li, L.; Fang, Q. Synthesis of a Carrageenan–Iron Complex and Its Effect on Flame Retardancy and Smoke Suppression for Waterborne Epoxy. Polymers 2019, 11, 1677. [Google Scholar] [CrossRef] [Green Version]
- Palin, L.; Rombolà, G.; Milanesio, M.; Boccaleri, E. The Use of POSS-Based Nanoadditives for Cable-Grade PVC: Effects on Its Thermal Stability. Polymers 2019, 11, 1105. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Tawiah, B.; Shi, Y.; Cai, S.; Rao, X.; Liu, C.; Yang, Y.; Yang, F.; Yu, B.; Liang, Y.; et al. Highly Effective Flame-Retardant Rigid Polyurethane Foams: Fabrication and Applications in Inhibition of Coal Combustion. Polymers 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Du, Y.; Wang, D.; Qin, B. Recent Progress in Polymer-Containing Soft Matters for Safe Mining of Coal. Polymers 2019, 11, 1706. [Google Scholar] [CrossRef] [Green Version]
- Czarnecka-Komorowska, D.; Sterzyński, T.; Dutkiewicz, M.; Maciejewski, H. POM / POSS Polyoxymethylene Composite with Increased Impact Strength and Thermal Stability, Method of Its Preparation and Application; (orginal title in Polish: Kompozyt polioksymetylenu POM/POSS o podwyższonej udarności i stabilności termicznej, sposób jego otrzymywania oraz zastosowanie); Poznan University of Technology: Poznan, Poland, 2018; p. 407468. [Google Scholar]
- Czarnecka-Komorowska, D.; Sterzynski, T. Effect of Polyhedral Oligomeric Silsesquioxane on the Melting, Structure, and Mechanical Behavior of Polyoxymethylene. Polymers 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnecka-Komorowska, D.; Mencel, K. Modification of polyamide 6 and polyoxymethylene with [3-(2-aminoethyl) amino] propyl-heptaisobutyl-polysilsesquioxane nanoparticles. Przem. Chem. 2014, 93, 392. [Google Scholar]
- Czarnecka-Komorowska, D.; Sterzynski, T.; Dutkiewicz, M. Polyoxymethylene/Polyhedral Oligomeric Silsesquioxane Composites: Processing, Crystallization, Morphology and Thermo-Mechanical Behavior. Int. Polym. Process. 2016, 31, 598–606. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Sterzynski, T.; Marciniec, B.; Dutkiewicz, M.; Szubert, K.; Galina, H.; Heneczkowski, M.; Oleksy, M.; Oliwa, R. Polyoxymethylene Composite with Reduced Formaldehyde Emission and Method for Making and Use Thereof. European Patent EP2886569A1, 2015. Available online: https://patents.google.com/patent/EP2886569A1/en (accessed on 10 August 2020).
- Lichtenhan, J.D.; Pielichowski, K.; Blanco, I. POSS-Based Polymers. Polymers 2019, 11, 1727. [Google Scholar] [CrossRef] [Green Version]
- Scott, D.W. Thermal Rearrangement of Branched-Chain Methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar] [CrossRef]
- Feher, F.J. Polyhedral Oligometallasilsesquioxanes (POMSS) as Models for Silica-Supported Transiton-Metal Catalysts: Synthesis and Characterization of (C5Me5)Zr[(Si7O12)(c-C6H11)7]. J. Am. Chem. Soc. 1986, 108, 3850–3852. [Google Scholar] [CrossRef]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Xu, F.; Li, G. Combustion Performance and Thermal Stability of Basalt Fiber-Reinforced Polypropylene Composites. Polymers 2019, 11, 1826. [Google Scholar] [CrossRef] [Green Version]
- Barczewski, M.; Sałasińska, K.; Kloziński, A.; Skórczewska, K.; Szulc, J.; Piasecki, A. Application of the Basalt Powder as a Filler for Polypropylene Composites with Improved Thermo-Mechanical Stability and Reduced Flammability. Polym. Eng. Sci. 2019, 59, E71–E79. [Google Scholar] [CrossRef]
- Sheng, X.; Li, S.; Zhao, Y.; Zhai, D.; Zhang, L.; Lu, X. Synergistic Effects of Two-Dimensional MXene and Ammonium Polyphosphate on Enhancing the Fire Safety of Polyvinyl Alcohol Composite Aerogels. Polymers 2019, 11, 1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, M.; Kinoshita, T. Aramid Fiber Cord for Power Transmission Belt and Method of Manufacturing the Same. United States Patent US5230667A, 1993. Available online: https://patents.google.com/patent/US5230667A/en (accessed on 23 October 2019).
- Gawdzińska, K.; Chybowski, L.; Przetakiewicz, W. Study of Thermal Properties of Cast Metal-Ceramic Composite Foams. Arch. Foundry Eng. 2017, 17, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.D.; Gilman, J.W.; VanderHart, D.L. Processing degradation of polyamide 6/montmorillonite clay nanocomposites and clay organic modifier. Polym. Degrad. Stab. 2003, 79, 111–121. [Google Scholar] [CrossRef]
- Brecoflex cataloque.pdf. Available online: https://www.brecoflex.com/wp-content/uploads/2016/07/B210.pdf (accessed on 19 October 2019).
- Chiorino cataloque.pdf. Available online: https://www.chiorino.com/pdf/CG35_LL2_EN.pdf (accessed on 19 October 2019).
- Nitta Corporation_cataloque.pdf. Available online: https://www.nittacorporation.com/en/pdf (accessed on 19 October 2019).
- PN-EN ISO 1183-1. Plastics—Methods for Determining the Density of Non-Porous Plastics—Part 1: Immersion Method, Liquid Pycnometer Method and Titration Method; Polish Committee for Standardization: Warsaw, Poland, 2005. [Google Scholar]
- PN-EN ISO 868. Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness); Polish Committee for Standardization: Warsaw, Poland, 2003. [Google Scholar]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Abate, L.; Asarisi, V.; Blanco, I.; Cicala, G.; Recca, G. The influence of sulfonation degree on the thermal behaviour of sulfonated poly(arylene ethersulfone)s. Polym. Degrad. Stab. 2010, 95, 1568–1574. [Google Scholar] [CrossRef]
- Polish Standard. PN-B-02855 Study of the Release of Toxic Products Used and the Combustion of Materials (In Polish: Badanie Wydzielania Toksycznych Produktów Rozkładu i Spalania Materiałów); Polish Standardization Committee: Warsaw, Poland, 1988. [Google Scholar]
- France Standard. NFx70-100 89: Fire Behaviour Tests—Analysis of Pyrolysis and Combustion Gases-Pipe Still Method; AFNOR: La Plaine Saint-Denis, France, 2015. [Google Scholar]
- German Standard. DIN 53436: Generation of Thermal Decomposition Products from Materials for Their Analytic-Toxicological Testing; German Institute for Standardisation: Berlin, Germany, 1 January 2015. [Google Scholar]
- Russian Standard: GOST 12.1.044-89: Occupational Safety Standards System. Fire and Explosion Hazard of Substances and Materials. In Nomenclature of Indices and Methods of Their Determination; GOST: Moscow, Russia, 1991.
- Research Stand. Available online: http://sychta.eu/pn-b-02855.html (accessed on 21 January 2020).
- Herrera, M.; Matuschek, G.; Kettrup, A. Thermal degradation of thermoplastic polyurethane elastomers (TPU) based on MDI. Polym. Degrad. Stab. 2002, 78, 323. [Google Scholar] [CrossRef]
- Półka, M. Toxicity analysis of thermal decomposition and combustion products obtained in selected epoxy materials (In Polish: Analiza toksyczności produktów rozkładu termicznego i spalania uzyskanych w wybranych materiałów epoksydowych). Bezpieczeństwo Tech. Pożarnicza 2010, 3, 73–81. [Google Scholar]
- Dobrzyńska, R. Influence of toxicity of thermal decomposition products and combustion of home furnishings materials on safe evacuation conditions (orginal title in Polish: Wpływ toksyczności produktów rozkładu termicznego i spalania materiałów wyposażenia wnętrz na warunki bezpiecznej ewakuacji). Pract. Nauk. Akad. Jana Długosza Częstochowie Tech. Inform. Inżynieria Bezpieczeństwa 2014, 2, 13–21. [Google Scholar]
- Dobrzyńska, R. Toxic Risk During Fire of Upholstered Furniture (In Polish: Zagrożenie Toksyczne Podczas Pożaru Mebli Tapicerowanych). Available online: http://rdobrzynska.zut.edu.pl/fileadmin/publikacje/Zagrozenie_toksyczne_podczas_pozaru_mebli_tapicerowanych.pdf (accessed on 21 January 2020).
- Regulation of the Minister of Infrastructure on the Technical Conditions to be Met by Buildings and Their Location. (Orginal Title in Polish: Rozporządzenie Ministra Infrastruktury w Sprawie Warunków Technicznych Jakim Powinny Odpowiadać Budynki i ich Usytuowanie). Available online: https://ec.europa.eu/growth/tools-databases/tris/en/search/?trisaction=search.detail&year=2017&num=326 (accessed on 21 January 2020).
- Irvine, D.J.; McCluskey, J.A.; Robinson, I.M. Fire hazards and some common polymers. Polym. Degrad. Stab. 2000, 67, 383–396. [Google Scholar] [CrossRef]
- Półka, M.; Piechocka, E. What is inside? (In Polish: Co czyha we wnętrzu?). Przegląd Pożarniczy 2008, 8, 28–31. [Google Scholar]
- Brennan, P. Victims and Survivours in Fatal Residential Building Fires. Fire Mater. 1999, 23, 305–310. [Google Scholar] [CrossRef]
- Guzewski, P.; Wróblewski, D.; Małozięć, D. Selected Problems of Fires and Their Effects (In Polish: Czerwona Księga Pożarów. Wybrane Problemy Pożarów Oraz ich Skutków); Tom 1: Józefów, Poland, 2016. [Google Scholar]
- Nagrodzka, M.; Małozięć, D. Fire resistant compounds application used in construction materials. (In Polish: Znaczenie środków ognioochronnych wykorzystywanych w materiałach stosowanych w budownictwie). Bezpieczeństwo Tech. Pożarnicza 2010, 2, 51–60. [Google Scholar]
- Porowski, R.; Kuźnicki, Z.; Małozięć, D.; Dziechciarz, A. Determination of Toxicity in Combustion Products-State of the Art. (In Polish: Oznaczanie toksyczności produktów spalania–przegląd stanu wiedzy). Bezpieczeństwo Tech. Pożarnicza 2018, 52, 82–100. [Google Scholar]
- Hirschler, M.M. Thermal analysis and flammability of polymers. Effect of halogen-metal additive systems. Eur. Polym. J. 1983, 19, 121. [Google Scholar] [CrossRef]
- Parcheta, P.; Głowińska, E.; Datta, J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020, 123, 109422. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Zavargo, Z.; Flyn, J.H.; Macknight, W.J. Thermal degradation of segmented polyurethanes. J. Appl. Polym. Sci. 1994, 51, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Yoshitake, N.; Furukawa, M. Thermal degradation mechanism of α,γ-diphenyl alkyl allophanate as a model polyurethane by pyrolysis-high-resolution gas chromatography/FT-IR. J. Anal. Appl. Pyrol. 1995, 33, 269–281. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Basfar, M.; Abdel Aziz, M. Comparison of thermal stability of sulfur, peroxide and radiation cured NBR and SBR vulcanizates. Polym. Degrad. Stab. 2000, 67, 319–323. [Google Scholar] [CrossRef]
- Corcuera, M.A.; Rueda, L.; Saralegui, A.; Martín, M.D.; Fernández-d’Arlas, B.; Mondragon, I.; Eceiza, A. Effect of diisocyanate structure on the properties and microstructure of polyurethanes based on polyols derived from renewable resources. J. Appl. Polym. Sci. 2011, 122, 3677–3685. [Google Scholar] [CrossRef]
- Marinović-Cincović, M.; Janković, B.; Jovanović, V.; Samaržija-Jovanović, S.; Marković, G. The kinetic and thermodynamic analyses of non-isothermal degradation process of acrylonitrile–butadiene and ethylene–propylene–diene rubbers. Compos. Part B Eng. 2013, 45, 321–332. [Google Scholar] [CrossRef]
- Matykiewicz, D. Biochar as an Effective Filler of Carbon Fiber Reinforced Bio-Epoxy Composites. Processes 2020, 8, 724. [Google Scholar] [CrossRef]









| Samples | Thickness, mm | Hardness, oShA | Density, g/cm3 |
|---|---|---|---|
| NE22 | 1.4 ± 0.3 | 82.2 ± 1.0 | 1.20 ± 0.04 |
| LL2 | 4.0 ± 0.1 | 76.8 ± 2.1 | 1.30 ± 0.07 |
| TC | 1.4 ± 0.2 | 78.8 ± 0.4 | 1.06 ± 0.03 |
| XH | 4.0 ± 0.1 | 73.8 ± 0.6 | 1.13 ± 0.05 |
| Product of Thermal Decomposition and Combustion | Value (g·m−3) |
|---|---|
| Carbon monoxide (CO) | 3.75 |
| Carbon dioxide (CO2) | 196.40 |
| Hydrogen cyanide (HCN) | 0.16 |
| Nitrogen dioxide (NO2) | 0.205 |
| Hydrogen chloride (HCl) | 1.00 |
| Sulfur dioxide (SO2) | 0.70 |
| WLC50SM | Label | Products of Thermal Decomposition and Combustion of Materials |
|---|---|---|
| 15 |  | very toxic |
| 15, 40 |  | toxic |
| 40 |  | moderately toxic |
| Identified Substances | NE22 | LL2 | TC | XH |
|---|---|---|---|---|
| SO2 | + | + | − | + |
| NO2 | − | + | − | + |
| NO | + | + | − | + |
| HCN | + | + | − | + |
| CO2 | + | + | + | + |
| CO | + | + | + | + |
| HCL | − | − | − | − |
| HBr | − | − | − | − |
| HF | − | − | − | − |
| Type of Belt | Value of Toxicometric Indicator WLC50SM [g/m3] | Material Classification |
|---|---|---|
| NE22 | 59.0 | moderately toxic |
| LL2 | 33.8 | toxic |
| TC | 195.0 | moderately toxic |
| XH | 46.8 | moderately toxic |
| Samples | T5% [°C] | T10% [°C] | T50% [°C] | DTG max rate (%/min) | Residue at 800 °C % |
|---|---|---|---|---|---|
| NE22 | 286.1 | 328.4 | 336.8 | 22.80/4.0 | 40 ± 1 |
| LL2 | 115.0 | 264.3 | 428.0 | 3.71/7.13 | 21 ± 1 |
| TC | 318.4 | 334.7 | 390.8 | 10.92 | 9 ± 3 |
| XH | 448.4 | 459.9 | 464.5 | 10.97 | 27 ± 2 |
| Samples | T1max/T2max 10 °C/min | T1max/T2max 20 °C/min | T1max/T2max 30 °C/min |
|---|---|---|---|
| NE22 | 312.5/416.0 | 373.0/418.7 | 385.0/424.1 |
| LL2 | 336.0/467.0 | 350.0/458.9 | 356.2/464.0 |
| TC | 360.0/403.4 | 375.0/420.8 | 387.0/427.1 |
| XH | 448.4 | 459.9 | 464.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawiec, P.; Warguła, Ł.; Małozięć, D.; Kaczmarzyk, P.; Dziechciarz, A.; Czarnecka-Komorowska, D. The Toxicological Testing and Thermal Decomposition of Drive and Transport Belts Made of Thermoplastic Multilayer Polymer Materials. Polymers 2020, 12, 2232. https://doi.org/10.3390/polym12102232
Krawiec P, Warguła Ł, Małozięć D, Kaczmarzyk P, Dziechciarz A, Czarnecka-Komorowska D. The Toxicological Testing and Thermal Decomposition of Drive and Transport Belts Made of Thermoplastic Multilayer Polymer Materials. Polymers. 2020; 12(10):2232. https://doi.org/10.3390/polym12102232
Chicago/Turabian StyleKrawiec, Piotr, Łukasz Warguła, Daniel Małozięć, Piotr Kaczmarzyk, Anna Dziechciarz, and Dorota Czarnecka-Komorowska. 2020. "The Toxicological Testing and Thermal Decomposition of Drive and Transport Belts Made of Thermoplastic Multilayer Polymer Materials" Polymers 12, no. 10: 2232. https://doi.org/10.3390/polym12102232
APA StyleKrawiec, P., Warguła, Ł., Małozięć, D., Kaczmarzyk, P., Dziechciarz, A., & Czarnecka-Komorowska, D. (2020). The Toxicological Testing and Thermal Decomposition of Drive and Transport Belts Made of Thermoplastic Multilayer Polymer Materials. Polymers, 12(10), 2232. https://doi.org/10.3390/polym12102232