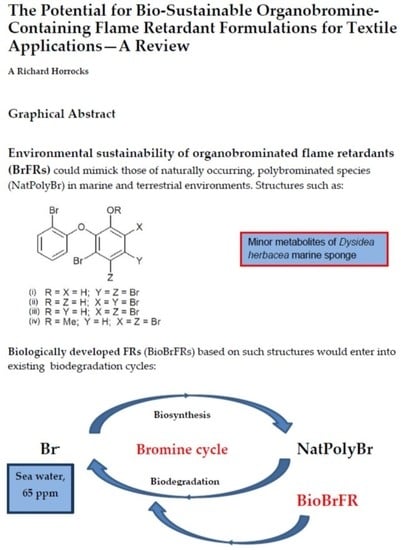
The Potential for Bio-Sustainable Organobromine-Containing Flame Retardant Formulations for Textile Applications—A Review
Abstract
:1. Introduction
2. Organobromine Flame Retardants—Environmental Challenges
- The high atomic weight of bromine, which ensures that BrFR molecules comprise typically >60% bromine, thereby reducing the total amount of flame retardant required.
- Acceptable levels of flame retardancy can usually be achieved with bromine levels of 5–10 wt%, which enables total BrFR contents to be in the range 9–15 wt%, much lower than for many phosphorus-containing species in which phosphorus contents are often <30 wt%.
- The carbon-bromine bond is strong enough to resist normal processing temperatures typically up to 250 °C or so, especially if it is an aromatic C–Br bond, while it breaks down into active bromine. radicals above 300 °C.
2.1. Current Alternatives to HBCD and DecaBDE
2.2. Effectiveness of BrFRs in Textile Coatings and Back-Coatings
- They function on any textile substrate, including the many fibre blends used in the industry, because of their predominant gas phase, flame quenching property and so are independent of the type of fibre present.
- The initial release of active Br˙ radicals and into the gas phase following thermal rupture of C–Br bonds within the flame retardant is simply represented by:Subsequent interactions with ATO produces volatile antimony-bromine species such as SbBr3 and antimony oxybromides [38] (see also Section 3.1) enable their diffusion to the front face of the fabric and extinguish any potential flame generation. In addition, these species may diffuse into an underlying substrate such as a polyurethane foam or other filling and extinguish any flames arising there. Thus the ATO synergized BrFR combination may be considered to provide both effective flame retardancy and the formation of a virtual barrier to flame penetration. Figure 1 presents a schematic representation of their mode of action.BrFR → Br˙ + FR˙
- The presence of a char-forming resin component minimizes the effect of a thermoplastic fibre-containing face fabric, such as polyester, in that any hole size is minimized as well as allowing the released bromine radicals to quench any underlying PU foam ignition.
2.3. Attempts to Replace BrFRs in Textile Back-Coatings
2.3.1. Reducing the BrFR Concentrations
2.3.2. Effectiveness of Phosphorus
2.3.3. The Sensitisation of Decomposition or Flame Retarding Efficiency of Phosphorus-Based Systems
2.3.4. The Introduction of a Volatile and Possible Vapour-Phase Active, Phosphorus-Containing Flame Retardants (PFRs)
2.4. “Biobromine” as an Environmentally Acceptable Source of BrFRs
2.4.1. Examples of Naturally Occurring Polybrominated Aromatic Compounds
2.4.2. Factors Influencing Biodegradability of Brominated Flame Retardants
3. Organobromine Flame Retardant Synergists
3.1. Antimony III Oxide
- the effectiveness of the Sb2O3/neoprene combination in regard to HCl generation;
- the identification of an optimum Sb2O3/Cl ratio equivalent to antimony oxyhalide, SbOCl, formation as an intermediate;
- the use of additional boric acid, zinc borate or an ammonium phosphate as afterglow retardants; and
- the combination of the neoprene/Sb2O3 combination alone was wash durable, but not afterglow retardant.
3.2. Zinc Stannates
3.3. Metal Tungstates
4. Conclusions
Funding
Conflicts of Interest
References
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate ester flame retardants: Are they a regrettable substitution for polybrominated diphenyl ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Dumler, R.; Thoma, H.; Lenoir, D.; Hutzinger, O. PBDF and PBDD from the combustion of bromine containing flame retarded polymers: A survey. Chemosphere 1989, 19, 2023–2031. [Google Scholar] [CrossRef]
- McAllister, D.L. Brominated flame retardants: Current issues and future prospects. In Flame Retardants 92; Interscience Publications: London, UK, 1992; pp. 149–155. [Google Scholar]
- Zhang, M.; Buekens, A.; Li, X. Brominated flame retardants and the formation of dioxins and furans in fires and combustion. J. Hazard. Mater. 2016, 304, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Wakelyn, P.J. Environmentally friendly flame retardant textiles. In Advances in Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 188–212. [Google Scholar]
- Horrocks, A.R. Flame retardant and environmental issues. In Update on Flame Retardant Textiles: State of the Art, Environmental Issues and Innovative Solutions; Alongi, J., Horrocks, A.R., Carosio, F., Malucelli, G., Eds.; Smithers Rapra: Shawbury, UK, 2013; pp. 207–238. [Google Scholar]
- Stapleton, H.M.; Misenheimer, J.; Hoffman, K.; Webster, T.F. Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014, 116, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, P.; Yan, X.; Zhu, Q.; Shi, J.; Liao, C.; Jiang, G. A review of environmental occurrence, fate, and toxicity of novel brominated flame retardants. Environ. Sci. Technol. 2019, 53, 13551–13569. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Syed, J.H.; Katsoyiannis, A.; Cincinelli, A.; Jones, K.C. Legacy and emerging flame retardants (FRs) in the freshwater ecosystem: A review. Environ. Res. 2017, 152, 26–42. [Google Scholar] [CrossRef]
- Brits, M.; de Vos, J.; Weiss, J.M.; Rohwer, E.R.; de Boer, J. Critical review of the analysis of brominated flame retardants and their environmental levels in Africa. Chemosphere 2016, 164, 174–189. [Google Scholar] [CrossRef]
- Yu, G.; Bu, Q.; Cao, Z.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef]
- Borsolini, S.; Baroni, D.; Martellini, T.; Pala, N.; Cincinelli, A. PBDEs and PCBs in terrestrial ecosystems of the Victoria Land, Antarctica. Chemosphere 2019, 231, 233–239. [Google Scholar] [CrossRef]
- de Wit, C.A.; Herzke, D.; Vorkamp, K. Brominated flame retardants in the Arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885–2918. [Google Scholar] [CrossRef]
- Ling, S.; Huang, K.; Tariq, M.; Wang, Y.; Chen, X.; Lin, K.; Zhou, B. Photodegradation of novel brominated flame retardants (NBFRs) in a liquid system: Kinetics and photoproducts. Chem. Eng. J. 2019, 362, 938–946. [Google Scholar] [CrossRef]
- Wang, R.; Tang, T.; Feng, S.; Xie, J.; Tao, X.; Huang, K.; Zou, M.; Yin, H.; Dang, Z.; Lu, G. Debromination of polybrominated diphenyl ethers (PBDEs) and their conversion to polybrominated dibenzofurans (PBDFs) by UV light: Mechanisms and pathways. J. Hazard. Mater. 2018, 354, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nadjia, L.; Abdelkader, E.; Ulrich, M.; Bekka, A. Spectroscopic behavior of Saytex 8010 under UV-visible light and comparative thermal study with some flame bromine retardant. J. Photochem. Photobiol. A Chem. 2014, 275, 96–102. [Google Scholar] [CrossRef]
- Tang, S.; Yin, H.; Chen, S.; Peng, H.; Change, J.; Liu, Z.; Dang, Z. Aerobic degradation of BDE-209 by Enterococcus casseliflavus: Isolation, identification and cell changes during degradation process. J. Hazard. Mater. 2016, 308, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Waaijers, S.L.; Parsons, J.R. Biodegradation of brominated and organophosphorus flame retardants. Curr. Opin. Biotechnol. 2016, 38, 14–23. [Google Scholar] [CrossRef]
- Weil, E.D. Synergists, adjuvants and antagonists in flame retardant systems. In Fire Retardancy of Polymeric Materials; Grand, A.F., Wilkie, C.A., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 120–122. [Google Scholar]
- Antimony, Global Industry, Markets & Outlook 2018, Report Roskill. Available online: https://roskill.com/market-report/antimony/ (accessed on 24 August 2020).
- Tanaka, T.; Terakado, O.; Hirasawa, M. Thermochemical approach for screening of alternative metal oxides as a flame retardant of modacrylic fiber. High Temp. Mater. Proccesses 2017, 36, 233–242. [Google Scholar] [CrossRef]
- National Research Council. Antimony trioxide. In Toxicological Risks of Selected Flame-Retardant Chemicals; National Academies Press: Washington, DC, USA, 2000; pp. 229–261. [Google Scholar]
- US Department of Health and Human Services. Report on Carcinogens Monograph on Antimony Trioxide. 2018. Available online: https://ntp.niehs.nih.gov/ntp/roc/monographs/antimony_final20181019_508.pdf (accessed on 24 August 2020).
- Georlette, P. Applications of halogen flame retardants. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2000; pp. 264–292. [Google Scholar]
- Horrocks, A.R. Overview of traditional flame-retardant solutions (including coating and back-coating). In Update on Flame Retardant Textiles: State of the Art, Environmental Issues and Innovative Solutions; Alongi, J., Horrocks, A.R., Carosio, F., Malucelli, G., Eds.; Smithers Rapra: Shawbury, UK, 2013; pp. 152–171. [Google Scholar]
- Zhang, S.; Horrocks, A.R. A review of flame retardant polypropylene fibres. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Clark, A. The Consumer Protection Act 1987. Mod. Law Rev. 1987, 50, 614–622. [Google Scholar]
- Van Esch, G.J. Brominated Diphenyl Ethers. In Environmental Health Criteria; WHO World Health Organisation: Geneva, Switzerland, 1994. [Google Scholar]
- European Union Risk Assessment Draft Report for Hexabromocyclododecane, European Chemicals Bureau (October 2008). Available online: https://echa.europa.eu/documents/10162/661bff17-dc0a-4475-9758-40bdd6198f82 (accessed on 24 August 2020).
- United Nations. Stockholm Convention on Persistent Organic Pollutants Stockholm, 22 May 2001, Amendment to Annex A, 26 November 2013, Listing of Hexabromocyclododecane. Available online: https://treaties.un.org/doc/Publication/CN/2013/CN.934.2013-Eng.pdf (accessed on 24 August 2020).
- Stevens, G.C.; Emsley, A.; Lim, A.; Williams, P. The benefits of fire measures in the UK and Europe from a consideration of UK and International Statistics. In Proceedings of the Flame Retardants 2006, London, UK, 14 February 2006; Interscience Publications: London, UK, 2006; pp. 235–246. [Google Scholar]
- European Chemicals Bureau. European Union Risk Assessment Report for bis(pentabromodiphenyl) Ether, July 2002 (closed 26th May 2004). Available online: https://echa.europa.eu/documents/10162/6434698/orats_final_rar_bispentabromophenylether_en.pdf (accessed on 24 August 2020).
- EU Commission Regulation (EU) 2017/227 of 9 February 2017 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards bis(Pentabromophenyl)ether; EU Commission: Brussels, Belgium, 2007.
- Dungey, S.; Akintoye, L. Environmental Risk Evaluation Report: 1,1′(Ethane-1,2-diyl)bis[Penta-Bromobenzene]; Environment Agency: Bristo, UK, 2007. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/290840/scho0507bmor-e-e.pdf (accessed on 24 August 2020).
- Kierkegaard, A.; Björklund, J.; Fridén, U. Identification of the flame retardant decabromodiphenyl ethane in the environment. Environ. Sci. Technol. 2004, 38, 3247–3253. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame retardants in commercial use or development for textiles. J. Fire Sci. 2008, 26, 243–281. [Google Scholar] [CrossRef]
- Sühringa, R.; Busch, F.; Fricke, N.; Kötke, D.; Wolschke, H.; Ebinghaus, R. Distribution of brominated flame retardants and dechloranes between sediments and benthic fish—A comparison of a freshwater and marine habitat. Sci. Total Environ. Part A 2016, 542, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hastie, J.W. Molecular basis of flame inhibition. J. Res. Natl. Bur. Stand. 1973, 77, 733–754. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, A.R. Recent developments in flame retardant textile finishes. In Textile Finishing, Recent Developments and Future Trends; Mittal, K.L., Bahners, T., Eds.; Wiley-Scrivener: New York, NY, USA, 2017; pp. 69–126. [Google Scholar]
- Dombrowski, R. Flame retardants for textile coatings. J. Coat. Fabr. 1996, 25, 224–238. [Google Scholar] [CrossRef]
- Conway, R. Coating of textiles. In Handbook of Technical Textiles, Vol. 1 Technical Textile Processes; Horrocks, A.R., Anand, S.C., Eds.; Woodhead Publishing Series in Textiles; Elsevier: Amsterdam, The Netherlands, 2016; pp. 211–230. [Google Scholar]
- Davies, P.J.; Horrocks, A.R. Optimisation of flame retardant textile coating parameters by factorial analysis. J. Coat. Fabr. 1999, 29, 118–138. [Google Scholar]
- Wang, M.Y.; Horrocks, A.R.; Horrocks, S.; Hall, M.E.; Pearson, J.S.; Clegg, S. Flame retardant textile back-coatings. Part 1: Antimony-halogen system interactions and the effect of replacement by phosphorus-containing agents. J. Fire Sci. 2000, 18, 265–294. [Google Scholar] [CrossRef]
- Weil, E.D. Additivity, synergism and antagonism in flame retardancy. In Flame Retardancy of Polymeric Materials, Volume 3; Kuryla, W.C., Papa, A.J., Eds.; Marcel Dekker: New York, NY, USA, 1975; pp. 185–243. [Google Scholar]
- Eivazi, S. Novel Environmentally Sustainable Surface Coatings for Textiles. Ph.D. Thesis, University of Bolton, Bolton, UK, 2018. [Google Scholar]
- Horrocks, A.R. Flame retardant/resistant coatings and laminates. In Advances in Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 159–183. [Google Scholar]
- Horrocks, A.R.; Wang, M.Y.; Hall, M.E.; Sunmonu, F.; Pearson, J.S. Flame retardant textile back-coatings. Part 2: Effectiveness of phosphorus-containing retardants in textile back-coating formulations. Polym. Int. 2000, 49, 1079–1091. [Google Scholar] [CrossRef]
- Lewin, M.; Endo, M. Catalysis of intumescent flame retardancy of polypropylene by metallic compounds. In Proceedings of the American Chemical Society Division of Polymeric Materials: Science and Engineering, Volume 83, American Chemical Society, Fall Meeting, Washington, DC, USA, 20–24 August 2000; pp. 38–39. [Google Scholar]
- Lewin, M.; Endo, M. Catalysis of intumescent flame retardancy of polypropylene by metallic compounds. Polym. Adv. Technol. 2003, 14, 3–11. [Google Scholar] [CrossRef]
- Davies, P.J.; Horrocks, A.R.; Alderson, A. The sensitisation of thermal decomposition of APP by selected metal ions and their potential for improved cotton fabric flame retardancy. Polym. Degrad. Stab. 2005, 88, 114–122. [Google Scholar] [CrossRef]
- Hastie, J.W.; Bonnell, D.W. Molecular Chemistry of Inhibited Combustion Systems; Report NBSIR 80-2169; National Bureau of Standards: Gaithersburg, MD, USA, 1980. [Google Scholar]
- Horrocks, A.R.; Davies, P.; Alderson, A.; Kandola, B. The potential for volatile phosphorus-containing flame retardants in textile back-coatings. J. Fire Sci. 2007, 25, 523–540. [Google Scholar] [CrossRef]
- Horrocks, A.R. Nanocomposites II: Potential applications for nanocomposite-based flame retardant systems. In Advances in Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 124–158. [Google Scholar]
- Alongi, J.; Carosio, F.; Malucelli, G. Smart (nano) coatings. In Update on Flame Retardant Textiles: State of the Art, Environmental Issues and Innovative Solutions; Alongi, J., Horrocks, A.R., Carosio, F., Malucelli, G., Eds.; Smithers Rapra: Shawbury, UK, 2013; pp. 257–312. [Google Scholar]
- Vasiljević, J.; Jerman, I.; Jakša, G.; Alongi, J.; Malucelli, G.; Zorko, M.; Tomšič, B.; Simončič, B. Functionalization of cellulose fibres with DOPO-polysilsesquioxane flame retardant nanocoating. Cellulose 2015, 22, 1893–1910. [Google Scholar] [CrossRef]
- Oner, N.; Mete, G. Development of water-, oil-repellent and flame retardant cotton fabrics by organic-inorganic hybrid materials. J. Text. Inst. 2016, 107, 1463–1477. [Google Scholar] [CrossRef]
- Mallucelli, G. Sol-Gel and layer-by-layer coatings for flame-retardant cotton fabrics: Recent advances. Coatings 2020, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Alongi, J.; Carosio, F.; Malucelli, G. Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: Flammability and combustion behaviour. Cellulose 2012, 19, 1041–1050. [Google Scholar] [CrossRef]
- Li, Y.C.; Mannen, S.; Morgan, A.B.; Chang, S.C.; Yang, Y.H.; Condon, B.; Grunlan, J.C. Intumescent all-polymer multilayer nanocoating capable of extinguishing flame on fabric. Adv. Mater. 2011, 23, 3926–3931. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Few durable layers suppress cotton combustion due to joint combination of layer by layer assembly and UV curing. RSC Adv. 2015, 5, 71482–71490. [Google Scholar] [CrossRef]
- Mateos, A.J.; Cain, A.A.; Grunlan, J.C. Deposition of flame retardant and conductive nanocoatings on fabric. Ind. Eng. Chem. Res. 2014, 53, 6409–6416. [Google Scholar] [CrossRef]
- Cain, A.A.; Murray, S.; Holder, K.M.; Nolen, C.R.; Grunlan, J.C. Intumescent nanocoating extinguishes flame on fabric using aqueous polyelectrolyte complex deposited in single step. Macromol. Mater. Eng. 2014, 299, 1180–1187. [Google Scholar] [CrossRef]
- Leistner, M.; Abu-Odeh, A.A.; Rohmer, S.C.; Grunlan, J.C. Water-based chitosan/melamine polyphosphate multilayer nanocoating that extinguishes fire on polyester-cotton fabric. Carbohydr. Polym. 2015, 130, 227–232. [Google Scholar] [CrossRef]
- Haile, M.; Leistner, M.; Sarwar, O.; Toler, C.M.; Henderson, R.; Grunlan, J.C. A wash-durable polyelectrolyte complex that extinguishes flames on polyester-cotton fabric. RSC Adv. 2016, 6, 33998–34004. [Google Scholar] [CrossRef]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Jama, C.; Queédeé, A.; Goudmand, P.; Dessaux, O.; Le Bras, M.; Delobel, R.; Bourbigot, S.; Gilman, J.W. Fire retardancy and thermal stability of materials coated by organosilicon thin films using a cold remote plasma process. ACS Symp. Ser. 2001, 797, 200–213. [Google Scholar]
- Horrocks, A.R.; Nazaré, S.; Masood, R.; Kandola, B.K.; Price, D. Surface modification of fabrics for improved flash-fire resistance using atmospheric pressure plasma. Polym. Adv. Technol. 2011, 22, 22–29. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Eivazi, S.; Ayesh, M.; Kandola, B.K. Environmentally sustainable flame retardant surface treatments for textiles: The potential of a novel atmospheric plasma/UV laser technology. Fibres 2018, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Gribble, G.W. The diversity of naturally produced organohalogens. Chemosphere 2003, 52, 289–297. [Google Scholar] [CrossRef]
- Falkner, D. Brominated marine natural products. In Bromine Compounds, Chemistry and Its Applications; Price, D., Iddon, B., Wakefield, B.J., Eds.; Elsevier: Amsterdam, NL, USA, 1988; pp. 121–144. [Google Scholar]
- Ladaki, M.; Szwarc, M. Studies of the variations in bond dissociation energies of aromatic compounds I. Mono-bromo-aryles. Proc. R. Soc. A Math. Phys. Eng. Sci. 1953, 219, 341–352. [Google Scholar]
- Kazakbayeva, Z.; Zhumagali, S.; Mahboob, A.; O’Reilly, S.J. Homolytic C–Br bond dissociation energies obtained by means of the G4 thermochemical protocol. Chem. Data Collect. 2016, 2, 43–48. [Google Scholar] [CrossRef]
- Hewson, W.D.; Hager, L.P. Bromoperoxidases and halogenated lipids in marine algae. J. Phycol. 1980, 16, 340–345. [Google Scholar] [CrossRef]
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep. 1999, 16, 155–198. [Google Scholar] [CrossRef]
- Handayani, D.; Edrada, R.A.; Proksch, P.; Wray, V.; Witte, L.; Van Soest, R.W.M. Four New Bioactive Polybrominated Diphenyl Ethers of the Sponge Dysidea herbacea from West Sumatra, Indonesia. J. Nat. Prod. 1997, 60, 1313–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden, B.F.; Towerzey, L.; Junk, P.C. A new brominated diphenyl ether from the marine sponge Dysidea herbacea. Aust. J. Chem. 2000, 53, 299–301. [Google Scholar] [CrossRef]
- Agrawal, M.S.; Bowden, B.F. Marine sponge revisited: Another brominated diphenyl ether. Mar. Drugs 2005, 3, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Leri, A.C.; Hakala, J.A.; Marcus, M.A.; Lanzirotti, A.; Reddy, C.M.; Myneni, S.C.B. Natural organobromine in marine sediments: New evidence of biogeochemical Br cycling. Glob. Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef] [Green Version]
- Berg, R.D.; Solomon, E.A. Geochemical constraints on the distribution and rates of debromination in the deep subseafloor biosphere. Geochim. Cosmochim. Acta 2016, 174, 30–41. [Google Scholar] [CrossRef]
- Christiansson, A.; Eriksson, J.; Teclechiel, D.; Bergman, A. Identification and quantification of products formed via photolysis of decabromodiphenyl ether. Environ. Sci. Pollut. Res. 2009, 16, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Altarawneh, M.; Siddique, K.; Conesa, J.A.; Ortuño, N.; Dlugogorski, B.Z. Photodecomposition properties of brominated flame retardants (BFRs). Ecotoxicol. Environ. Saf. 2020, 192, 110272. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.; Nachev, M.; Klein, J.; Köster, D.; Schmitz, O.J.; Schmidt, T.C.; Sures, B. Degradation of the polymeric brominated flame retardant “polymeric FR” by heat and UV exposure. Environ. Sci. Technol. 2019, 53, 1453–1462. [Google Scholar] [CrossRef] [Green Version]
- Kock, C.; Sures, B. Degradation of brominated polymeric flame retardants and effects of generated decomposition products. Chemosphere 2019, 227, 329–333. [Google Scholar] [CrossRef]
- Vonderheide, A.P.; Mueller-Spitz, S.R.; Meija, J.; Welsh, G.L.; Mueller, K.E.; Kinkle, B.K.; Shann, J.R.; Caruso, J.A. Rapid breakdown of brominated flame retardants by soil microorganisms. J. Anal. At. Spectrom. 2006, 21, 1232–1239. [Google Scholar] [CrossRef]
- Gerecke, A.C.; Giger, W.; Hartmann, P.C.; Heeb, N.V.; Kohler, H.P.E.; Schmid, P.; Zenegg, M.; Kohler, M. Anaerobic degradation of brominated flame retardants in sewage sludge. Chemosphere 2006, 64, 311–317. [Google Scholar] [CrossRef]
- Davis, J.W.; Gonsior, S.; Marty, G.; Ariano, J. The transformation of hexabromocyclododecane in aerobic and anaerobic soils and aquatic sediments. Water Res. 2005, 39, 1075–1084. [Google Scholar] [CrossRef]
- Davis, J.W.; Gonsior, S.J.; Markham, D.A.; Friederich, U.; Hunziker, R.W.; Ariano, J.M. Biodegradation and product identification of [14C] hexabromocyclodedecane in wastewater sludge and freshwater aquatic sediment. Environ. Sci. Technol. 2006, 40, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.H.; Wang, R.; Peng, Y.H.; Chou, T.H.; Li, Y.J.; Shih, Y.H. Biodegradation of hexabromocyclododecane by Rhodopseudomonas palustris YSC3 strain: A free-living nitrogen-fixing bacterium isolated in Taiwan. Chemosphere 2020, 246, 125621. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Peng, P.; Yu, Z.; Huang, W.; Zhong, Y. Reductive transformation of hexabromocyclododecane (HBCD) by FeS. Water Res. 2016, 101, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yu, Y.; Yang, Y.; Li, G.; An, T. A new advance in the potential exposure to “old” and “new” halogenated flame retardants in the atmospheric environments and biota: From occurrence to transformation products and metabolites. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1935–1983. [Google Scholar] [CrossRef]
- Pan, L.; Sun, J.; Wu, X.; Wei, Z.; Zhu, L. Transformation of hydroxylated and methoxylated 2,2′,4,4′,5-brominated diphenyl ether (BDE-99) in plants. J. Environ. Sci. 2016, 49, 197–202. [Google Scholar] [CrossRef]
- Leri, A.C.; Myneni, S.C.B. Natural organobromine in terrestrial ecosystems. Geochim. Cosmochim. Acta 2012, 77, 1–10. [Google Scholar] [CrossRef]
- Perkin, W.H. Metal Oxides as Flame Retardants to Cellulose. U.S. Patent 844,042, 2 December 1907. [Google Scholar]
- Little, R.W. (Ed.) Flame-Proofing Textile Fabrics; Reinhold Publishing Corporation: New York, NY, USA, 1947. [Google Scholar]
- Coppick, S. Metallic oxide-chlorinated body type, a. fundamentals of processes. In Flame-Proofing Textile Fabrics; Little, R.W., Ed.; Reinhold Publishing Corporation: New York, NY, USA, 1947; pp. 239–247. [Google Scholar]
- Costa, L.; Goberti, P.; Paganetto, G.; Camino, G.; Sgarzi, P. Thermal behaviour of chlorine-antimony fire-retardant systems. Polym. Degrad. Stab. 1990, 30, 13–28. [Google Scholar] [CrossRef]
- Costa, L.; Paganetto, G.; Bertelli, G.; Camino, G. Thermal decomposition of antimony oxyhalides I Oxychlorides. J. Therm. Anal. 1990, 36, 1141–1153. [Google Scholar] [CrossRef]
- Bertelli, G.; Costa, L.; Fenza, S.; Marchetti, E.; Camino, G.; Locatelli, R. Thermal behaviour of bromine-metal fire retardant systems. Polym. Degrad. Stab. 1988, 20, 295–314. [Google Scholar] [CrossRef]
- Hastie, J.W. Mass spectrometric studies of flame inhibition. Combust Flame 1973, 21, 49–54. [Google Scholar] [CrossRef]
- Stec, A.A. Fire toxicity—The elephant in the room? Fire Saf. J. 2017, 19, 79–90. [Google Scholar] [CrossRef]
- Purser, D. Toxicity of fire retardants in relation to life safety and environmental hazards. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2001; pp. 69–127. [Google Scholar]
- Pasternak, M.; Zinn, B.T.; Browner, R.F. Studies of the chemical mechanism of smoke particulates formation during the combustion of chlorinated polymers. Combust. Sci. Technol. 1982, 28, 263–270. [Google Scholar] [CrossRef]
- Shen, K.K.; Kochesfahani, S.H.; Jouffret, F. Boron-based flame retardants and flame retardancy. In Fire Retardancy of Polymeric Materials; Wilkie, C.A., Morgan, A.B., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 207–237. [Google Scholar]
- National Research Council. Zinc borate. In Toxicological Risks of Selected Flame-Retardant Chemicals; National Academies Press: Washington, DC, USA, 2000; pp. 149–191. [Google Scholar]
- Sigma Aldrich. Zinc Borate. CAS No 10361-94-1, MSDS Datasheets. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=14470&brand=SIAL&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsial%2F14470%3Flang%3Den (accessed on 24 August 2020).
- Roskill Information Services. Antimony Outlook to 2030, 14th ed.; Roskill Information Services: London, UK, 2020. [Google Scholar]
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. Metal scarcity and sustainability, analyzing the necessity to reduce the extraction of scarce metals. Res. Conserv. Recycl. 2014, 93, 1–8. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Smart, G.; Price, D.; Kandola, B. Zinc stannates as alternative synergists in selected flame retardant systems. J. Fire Sci. 2009, 27, 495–521. [Google Scholar] [CrossRef]
- Cusack, P.A.; Monk, A.W.; Pearce, J.A.; Reynolds, S.J. An investigation of inorganic tin flame retardants which suppress smoke and carbon monoxide emission from burning brominated polyester resins. Fire Mater. 1989, 14, 23–29. [Google Scholar] [CrossRef]
- Yukihiko, N.; Yasushi, K.; Misao, H.; Yasunori, K.; Hirofumi, K. Flame Retardant Polyolefin Compound Having Low Smoking and Toxicity. U.S. Patent 5726231, 10 March 1998. [Google Scholar]
- Markezich, R.L.; Mundhenke, R.F. Bromine/chlorine synergism to flame retardant plastics and stability of flame retardant-polyamides. In Flame Retardants ’98; Interscience Communications: London, UK, 1998; pp. 93–102. [Google Scholar]
- Cusack, P.A. Tin chemicals as flame retardants. In Recent Advances in Flame Retardancy of Polymeric Materials, Vol. 2; Lewin, M., Kirshenbaum, B., Eds.; Business Communications Inc.: Norwalk, CT, USA, 1991; pp. 75–82. [Google Scholar]
- Jung, H.C.; Kim, W.N.; Lee, C.R.; Suh, K.S.; Kim, S.R. Properties of flame-retarding blends of polycarbonate and poly(acrylonitrile-butadiene-styrene). J. Polym. Eng. 1998, 18, 115–130. [Google Scholar] [CrossRef]
- Cusack, P.A.; Heer, M.S.; Monk, A.W. Zinc hydroxystannate: A combined flame retardant and smoke suppressant for halogenated polyesters. Polym. Degrad. Stab. 1991, 32, 177–190. [Google Scholar] [CrossRef]
- Andre, F.; Cusack, P.A.; Monk, A.W.; Seangpraserkij, S. The effect of zinc hydroxystannate and zinc stannate on the fire properties of polyester resins containing additive-type flame retardants. Polym. Degrad. Stab. 1993, 40, 267–273. [Google Scholar] [CrossRef]
- Bains, R.S.; Cusack, P.A.; Monk, A.W. A comparison of the fire retardant properties of zinc hydroxystannate and antimony trioxide in brominated polyester resins containing inorganic fillers. Eur. Polym. J. 1990, 26, 1221–1227. [Google Scholar] [CrossRef]
- Kicko-Walczak, E. Flame retarded halogenated unsaturated polyester resins. Thermal decomposition study. J. Polym. Eng. 2003, 23, 149–161. [Google Scholar] [CrossRef]
- Kicko-Walczak, E. Studies on the mechanisms of thermal decomposition of unsaturated polyester resins with reduced flammability. Polym. Polym. Comp. 2004, 12, 127–134. [Google Scholar] [CrossRef]
- Ismaeili, N. Mechanistic Study of Synergism of Inorganic Synergists with Flame Retardants. Ph.D. Thesis, University of Bolton, Bolton, UK, 2019. [Google Scholar]
- Weil, E.D.; Levchik, S. Current practice and recent commercial developments in flame retardancy of polyamides. J. Fire Sci. 2004, 22, 251–264. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K.; Price, D. The potential of metal oxalates as novel flame retardants and synergists for engineering polymers. Polym. Degrad. Stab. 2014, 110, 290–297. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Synthesis and thermal analytical screening of metal complexes as potential novel fire retardants in polyamide 6.6. Polym. Degrad. Stab. 2017, 144, 420–433. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Novel metal complexes as potential synergists with phosphorus-based flame retardants in polyamide 6.6. Polym. Degrad. Stab. 2020, 179, 109220. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Potential synergism between novel metal complexes and polymeric brominated flame retardants in polyamide 6.6. Polymers 2020, 12, 1543. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Smart, G.; Kandola, B.K.; Holdsworth, A.F.; Price, D. Zinc stannate interactions with flame retardants in polyamides; Part 1: Synergies with organobromine-containing flame retardants in polyamides 6 (PA6) and 6.6 (PA6.6). Polym. Degrad. Stab. 2012, 97, 2503–2510. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]





| Material | Commercial Name and Supplier |
|---|---|
| Acrylic co-polymer resin | Hycar T-91 (Lubrizol) |
| BrFR flame retardants: | Decabromodiphenyl Ether, DecaBDE (FR-1210, ICL), 83% Bromine |
| Polymeric brominated FR, PolymBr, 62% solids dispersion, ~32% Bromine | |
| Polymeric brominated FR + phosphorus-containing species, PolymBrP, 50% solids dispersion, ~16% Bromine | |
| Synergist | Antimony III oxide (ATO) |
| Cotton fabric | Woven, 250 g/m2 |
| Formulation | Fabric Damaged Length, mm | Foam Damaged Depth, mm |
|---|---|---|
| DecaBDE/ATO | 55 | 17 |
| PolymBr/ATO | 50 | 13 |
| PolymBrP/ATO | 195 | 20 |
| Antimony III Oxide, Sb2O3 | Zinc Hydroxystannate (ZHS), Zn Sn(OH)6 | Zinc Stannate (ZS), ZnSn03 |
|---|---|---|
| White powder | White powder | White powder |
| % Antimony 83.5 | % Tin 41.0–43.0 | % Tin 53.0–56.0 |
| Water solubility 0.001 g/100 mL | % Zinc 22.0–23.5 | % Zinc 26.2–27.5 |
| water, 25 °C | % Moisture 0.7 max | % Moisture 0.5 max |
| Average particle size 0.4–1.8 microns | Average particle size (d50) 1.4–2.2 microns | Average particle size (d50) 1.4–2.2. microns |
| Sample | Composition (%) | Flammability Parameters | |||||
|---|---|---|---|---|---|---|---|
| PA66 | MC * | PolyBrFR | LOI, Vol.% | PHRR, kW/m2 | TSR m2/m2 | RPHRR % | |
| PA66 | 100 | - | - | 22.6 | 1644 | 609 | - |
| BrPS | 90 | - | 10 | 22.9 | 1049 | 1821 | 36.2 |
| BrPBz | 90 | - | 10 | 22.3 | 1206 | 1447 | 26.6 |
| AlW ** | 95 | 5 | - | 23.0 | 1156 | 927 | 29.7 |
| SnW ** | 95 | 5 | - | 21.5 | 954 | 939 | 42.0 |
| ZnW ** | 95 | 5 | - | 22.0 | 1190 | 638 | 27.6 |
| AlW-BrPS | 85 | 5 | 10 | 23.3 | 999 | 1789 | 39.2 |
| AlW-BrPBz | 85 | 5 | 10 | 22.3 | 1174 | 1246 | 28.6 |
| SnW-BrPS | 85 | 5 | 10 | 26.7 | 546 | 1973 | 66.8 |
| SnW-BrPBz | 85 | 5 | 10 | 26.7 | 802 | 1766 | 51.2 |
| ZnW-BrPS | 85 | 5 | 10 | 26.2 | 485 | 949 | 70.5 |
| ZnW-BrPBz | 85 | 5 | 10 | 28.5 | 896 | 1186 | 45.5 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horrocks, A.R. The Potential for Bio-Sustainable Organobromine-Containing Flame Retardant Formulations for Textile Applications—A Review. Polymers 2020, 12, 2160. https://doi.org/10.3390/polym12092160
Horrocks AR. The Potential for Bio-Sustainable Organobromine-Containing Flame Retardant Formulations for Textile Applications—A Review. Polymers. 2020; 12(9):2160. https://doi.org/10.3390/polym12092160
Chicago/Turabian StyleHorrocks, A Richard. 2020. "The Potential for Bio-Sustainable Organobromine-Containing Flame Retardant Formulations for Textile Applications—A Review" Polymers 12, no. 9: 2160. https://doi.org/10.3390/polym12092160
APA StyleHorrocks, A. R. (2020). The Potential for Bio-Sustainable Organobromine-Containing Flame Retardant Formulations for Textile Applications—A Review. Polymers, 12(9), 2160. https://doi.org/10.3390/polym12092160