Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab
Abstract
:1. Introduction
2. Materials and Methods
2.1. Irradiation of Chitosanwith γ-Radiation
2.2. Determination of the Molecular Weight of Chitosan Using the Viscometric Method
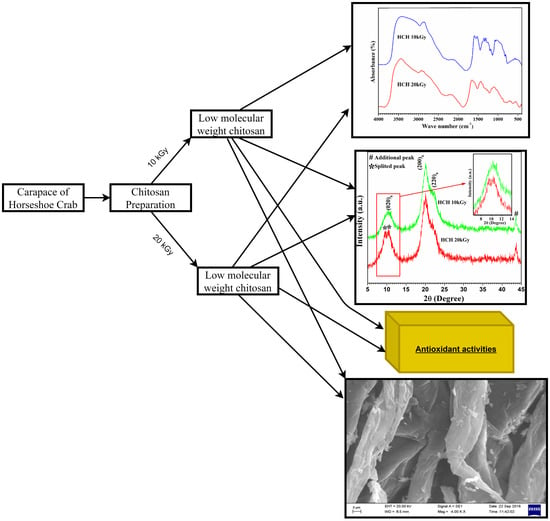
2.3. Characterization of Chitosan Samples
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity
2.4.2. Superoxide Anion Radical Scavenging Assay
2.4.3. Metal Ion Chelating Assay
2.4.4. Total Reducing Power Ability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Production of Low Molecular Weight Chitosan
3.2. Characterization of Horseshoe Crab Chitosan
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shirai, K.; Guerrero, I.; Huerta, S.; Saucedo, G.; Casillo, A.; Gonzalez, R.O.; Hall, G.M. Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation. Enzym. Microb. Technol. 2001, 28, 446–452. [Google Scholar] [CrossRef]
- Xu, Y.; Gallert, C.; Winter, J. Chitin purification from shrimp wastes by microbial deproteination and decalcification. Appl. Microbiol. Biotechnol. 2008, 79, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, O.; Elibol, M. Cocultivation of Lactococcuslactis and Teredinobacterturnirae for biological chitin extraction from prawn waste. Bioprocess. Biosyst. Eng. 2010, 33, 393–399. [Google Scholar] [CrossRef]
- Bautista, J.; Jover, M.; Guttierrez, J.F.; Corpas, R.; Cremades, O.; Fontiveros, E. Preparation of crayfish chitin by in situ lactic acid production. Process. Biochem. 2001, 37, 229–234. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Ravikumar, M.N.V.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci.Part C Polym. Rev. 2002, 42, 307–354. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez del Valle, M.G.; HernándezLópez, M.; AitBarka, E.; BosquezMolina, E.; Wilson, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop. Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Brzeski, M.M. Chitin and chitosan putting waste to good use. Infofish Int. 1987, 5, 31–33. [Google Scholar]
- Muzzarelli, R.A. Chitin and its derivatives: New trends of applied research. Carbohydr. Polym. 1983, 3, 53–75. [Google Scholar] [CrossRef]
- Pati, S.; Chatterji, A.; Dash, B.P. Chitosan from the carapace of Indian horseshoe crab (Tachypleusgigas, müller): Isolation and its characterization. Adv. Biores. 2016, 9, 52–64. [Google Scholar]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 21, 86–201. [Google Scholar] [CrossRef] [Green Version]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Nagamaniammai, G.; Chithra, M.; Udhaya Ganga, M. Study on the Effect of Prawn (Machrobrachuimrosenbergii) Chitosan Coating on Peeled Shallot (Allium ascalonicum). Curr. Res. Nutr. Food Sci. 2019, 7, 927–935. [Google Scholar] [CrossRef]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wasikiewicz, J.M.; Yoshii, F.; Nagasawa, N.; Wach, R.A.; Mitomo, H. Degradation of chitosan and sodium alginate by γ- radiation, sonochemical and ultraviolet methods. Radiat. Phys. Chem. 2005, 73, 287–295. [Google Scholar] [CrossRef]
- Chmielewski, A.G. Chitosan and radiation chemistry. Radiat. Phys. Chem. 2010, 79, 272–275. [Google Scholar] [CrossRef]
- Ciechanska, D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Text. East. Eur. 2004, 12, 69–72. [Google Scholar]
- Lopez-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltran, N.P.; Ornelas-Paz, J.D.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and antioxidant properties of chitosan polymers obtained from nontraditional Polybius henslowii sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Thomas, R.L. Antioxidative activity of chitosan with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.B. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Gryczka, U.; Gawronska, H.; Migdał, W.; Gawronski, S.W.; Chmielewski, A.G. Study on biological activity of chitosan after radiation processing. Nukleonika 2008, 53, S73–S76. [Google Scholar]
- Pati, S.; Jena, P.; Shahimi, S.; Nelson, B.R.; Acharya, D.; Dash, B.P.; Chatterji, A. Characterization dataset for pre-and post-irradiated shrimp waste chitosan. Data Brief 2020, 32, 106081. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Milas, M.; Le Dung, P. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.; Chen, Y.; Liu, S.; Zhao, H.; Gu, J. 60Co γ-ray Irradiation Crosslinking of Chitosan/Graphene Oxide Composite Film: Swelling, Thermal Stability, Mechanical, and Antibacterial Properties. Polymers 2018, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Domszy, J.G.; Roberts, G.A. Evaluation of infrared spectroscopic techniques for analysing chitosan. Makromol. Chem. Macromol. Chem. Phys. 1985, 186, 1671–1677. [Google Scholar] [CrossRef]
- Fernandez-Kim, S.O. Physicochemical and Functional Properties of Crawfish Chitosan as Affected by Different Processing Protocols. Ph.D. Thesis, Seoul National University, Seoul, Korea, 2004. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide, anions. Biochem. Pharmacol. 1988, 36, 317–322. [Google Scholar]
- Yen, G.C.; Duh, P.D.; Chuang, D.Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Yoksan, R.; Akashi, M.; Miyata, M.; Chirachanchai, S. Optimal gamma-ray dose and irradiation conditions for producing low-molecular-weight chitosan that retains its chemical structure. Radiat. Res. 2004, 161, 471–480. [Google Scholar] [CrossRef]
- Zainol, I.; Akil, H.M.; Mastor, A. Effect of γ-irradiation on the physical and mechanical properties of chitosan powder. Mater. Sci. Eng. C 2009, 29, 292–297. [Google Scholar] [CrossRef]
- Shen, K.; Hu, Q.; Wang, Z.; Qu, J. Effect of 60Co irradiation on the properties of chitosan rod. Mater. Sci. Eng. C 2011, 31, 866–872. [Google Scholar] [CrossRef]
- Taşkın, P.; Canısağ, H.; Şen, M. The effect of degree of deacetylation on the radiation induced degradation of chitosan. Radiat. Phys. Chem. 2014, 94, 236–239. [Google Scholar] [CrossRef]
- Yen, M.; Yang, J.; Mau, J. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Clark, G.L.; Smith, A.F. X-ray Diffraction Studies of Chitin, Chitosan, and Derivatives. J. Phys. Chem. 2002, 40, 863–879. [Google Scholar] [CrossRef]
- Younes, I.; Ghorbel-Bellaaj, O.; Chaabouni, M.; Rinaudo, M.; Souard, F.; Vanhaverbeke, C.; Jellouli, K.; Nasri, M. Use of a fractional factorial design to study the effects of experimental factors on the chitin deacetylation. Int. J. Biol. Macromol. 2014, 70, 385–390. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Phimolsiripol, Y. Antioxidant and Moisturizing Properties of Carboxymethyl Chitosan with Different Molecular Weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Zuhao, L.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Pati, S. Isolation of Chitosan from Carapace of Indian Horseshoe Crab (Tachypleusgigas; Müller) and Characterization of Its Antibacterial and Antioxidant Potentials. Ph.D. Thesis, Fakir Mohan University, Odisha, India, 2004. [Google Scholar]
- Yen, M.T.; Tseng, Y.H.; Li, R.C.; Mau, J.L. Antioxidant properties of fungal chitosan from shiitake stipes. LWT Food Sci. Technol. 2007, 40, 255–261. [Google Scholar] [CrossRef]
- Kuppusamy, S.K.; Karuppaiah, J. Invitro evaluation of free radical scavenging activity of chitosan. Int. J. Pharm. Life Sci. 2013, 4, 2685–2690. [Google Scholar]
- Prabu, K.; Natarajan, E. In vitro antibacterial and antioxidant activity of chitosan isolated from Podophthalmus vigil. J. Appl. Pharm. Sci. 2012, 2, 075–082. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chou, C.C. Antioxidant activities of water-soluble disaccharide chitosan derivatives. Food Res. Int. 2004, 37, 883–889. [Google Scholar] [CrossRef]
- Mat Zauki, N.A.; Satyanarayana, B.; Fairuz-Fozi, N.; Nelson, B.R.; Martin, M.B.; Akbar-John, B.; Chowdhury, A.J.K. Citizen science frontiers horseshoe crab population regain at their spawning beach in East Peninsular Malaysia. J. Environ. Manag. 2019, 232, 1012–1020. [Google Scholar] [CrossRef]
- Zauki, N.A.M.; Satyanarayana, B.; Fairuz-Fozi, N.; Nelson, B.R.; Martin, M.B.; Akbar-John, B.; Chowdhury, A.J.K. Horseshoe crab bio-ecological data from Balok, east coast peninsular Malaysia. Data Brief 2019, 22, 458–463. [Google Scholar] [CrossRef]
- John, B.A.; Nelson, B.R.; Sheikh, H.I.; Cheung, S.G.; Wardiatno, Y.; Dash, B.P.; Tsuchiya, K.; Iwasaki, Y.; Pati, S. A review on fisheries and conservation status of Asian horseshoe crabs. Biodivers. Conserv. 2018, 27, 3573–3598. [Google Scholar] [CrossRef]
- Pati, S.; Tudu, S.; Rajesh, A.; Biswal, G.C.; Chatterji, A.; Dash, B.P.; Samantaray, R. Manmade activities affected the breeding ground of horseshoe crab (Tachypleusgigas) along Balasore coast: Call for immediate conservation. E-Planet 2017, 15, 145–154. [Google Scholar]
- Nong, W.; Qu, Z.; Li, Y.; Owen, T.B.; Wong, A.Y.; Yip, H.Y.; Cao, J. Horseshoe crab genomes reveal the evolutionary fates of genes and microRNAs after three rounds (3R) of whole genome duplication. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnol. Bioprocess. Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pati, S.; Chatterji, A.; Dash, B.P.; Raveen Nelson, B.; Sarkar, T.; Shahimi, S.; Atan Edinur, H.; Binti Abd Manan, T.S.; Jena, P.; Mohanta, Y.K.; et al. Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers 2020, 12, 2361. https://doi.org/10.3390/polym12102361
Pati S, Chatterji A, Dash BP, Raveen Nelson B, Sarkar T, Shahimi S, Atan Edinur H, Binti Abd Manan TS, Jena P, Mohanta YK, et al. Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers. 2020; 12(10):2361. https://doi.org/10.3390/polym12102361
Chicago/Turabian StylePati, Siddhartha, Anil Chatterji, Bisnu Prasad Dash, Bryan Raveen Nelson, Tanmay Sarkar, Salwa Shahimi, Hisham Atan Edinur, Teh Sabariah Binti Abd Manan, Paramananda Jena, Yugal Kishore Mohanta, and et al. 2020. "Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab" Polymers 12, no. 10: 2361. https://doi.org/10.3390/polym12102361
APA StylePati, S., Chatterji, A., Dash, B. P., Raveen Nelson, B., Sarkar, T., Shahimi, S., Atan Edinur, H., Binti Abd Manan, T. S., Jena, P., Mohanta, Y. K., & Acharya, D. (2020). Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers, 12(10), 2361. https://doi.org/10.3390/polym12102361