Influence of Polylactide (PLA) Stereocomplexation on the Microstructure of PLA/PBS Blends and the Cell Morphology of Their Microcellular Foams
Abstract
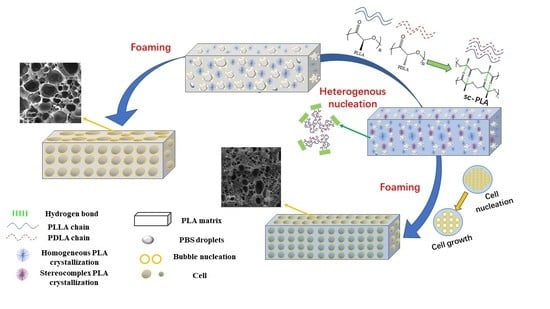
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparations
2.2. CO2 Saturation and Batch Foaming
2.3. Crystallization Behavior Analysis
2.4. Foam Morphology Characterization
3. Results and Discussion
3.1. Mixing Torque of the Blends
3.2. Crystallization Behavior
3.3. Effect of Poly (d-Lactide) (PDLA) on the Microstructure of Polylactide (PLA)/Poly (Butylene Succinate) (PBS) Blends
3.4. Effect of PBS on the Dynamic Mechanical Properties of PLA/PBS Blends
3.5. Foaming Morphology of PLA/PBS and PLA/PBS/PDLA Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stabil. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Q.; Chen, W.N.; Yang, J.; Bei, J.Z.; Wang, S.G. Biodegradable poly(L-lactide)-poly(ethylene glycol) multiblock copolymer: Synthesis and evaluation of cell affinity. Biomaterials 2003, 24, 2195–2203. [Google Scholar] [CrossRef]
- Zini, E.; Baiardo, M.; Armelao, L.; Scandola, M. Biodegradable polyesters reinforced with surface-modified vegetable fibers. Macromol. Biosci. 2004, 4, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Zolali, A.M.; Heshmati, V.; Favis, B.D. Ultratough Co-Continuous PLA/PA11 by Interfacially Percolated Poly(ether-b-amide). Macromolecules 2017, 50, 264–274. [Google Scholar] [CrossRef]
- Tasaka, F.; Ohya, Y.; Ouchi, T. One-pot synthesis of novel branched polylactide through the copolymerization of lactide with mevalonolactone. Macromol. Rapid Commun. 2001, 22, 820–824. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Mohanty, A.K.; Misra, M. Fully Biodegradable and Biorenewable Ternary Blends from Polylactide, Poly(3-hydroxybutyrate-co-hydroxyvalerate) and Poly(butylene succinate) with Balanced Properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Jing, Z.X.; Shi, X.T.; Zhang, G.C.; Lei, R.Y. Investigation of poly(lactide) stereocomplexation between linear poly(L-lactide) and PDLA-PEG-PDLA tri-block copolymer. Polym. Int. 2015, 64, 1399–1407. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E.; Gadzinowska, K.; Stasiak, M. Plasticization of poly(L-lactide) with poly(propylene glycol). Biomacromolecules 2006, 7, 2128–2135. [Google Scholar] [CrossRef]
- Standau, T.; Zhao, C.J.; Castellon, S.M.; Bonten, C.; Altstadt, V. Chemical Modification and Foam Processing of Polylactide (PLA). Polymers 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.T.; Zhang, G.C.; Siligardi, C.; Ori, G.; Lazzeri, A. Comparison of Precipitated Calcium Carbonate/Polylactic Acid and Halloysite/Polylactic Acid Nanocomposites. J. Nanomater. 2015, 2015, 905210. [Google Scholar] [CrossRef] [Green Version]
- Kakuta, M.; Hirata, M.; Kimura, Y. Stereoblock Polylactides as High-Performance Bio-Based Polymers. Polym. Rev. 2009, 49, 107–140. [Google Scholar] [CrossRef]
- Zhang, J.M.; Sato, H.; Tsuji, H.; Noda, I.; Ozaki, Y. Infrared spectroscopic study of CH3 center dot center dot center dot O=C interaction during poly(L-lactide)/poly(D-lactide) stereocomplex formation. Macromolecules 2005, 38, 1822–1828. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Stereocomplex Formation Between Enantiomeric Poly(Lactic Acid)S.10. Binary Blends from Poly(D-Lactide-Co-Glycolide) and Poly(L-Lactide-Co-Glycolide). J. Appl. Polym. Sci. 1994, 53, 1061–1071. [Google Scholar] [CrossRef]
- Yamane, H.; Sasai, K. Effect of the addition of poly(D-lactic acid) on the thermal property of poly(L-lactic acid). Polymer 2003, 44, 2569–2575. [Google Scholar] [CrossRef]
- Brzezinski, M.; Kost, B.; Wedepohl, S.; Socka, M.; Biela, T.; Calderon, M. Stereocomplexed PLA microspheres: Control over morphology, drug encapsulation and anticancer activity. Colloid Surf. B 2019, 184, 110544. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Feng, N.B.; Chang, F.; Wang, J.C.; Yuan, B.M.; Cheng, Y.L.; Liu, H.; Yu, J.K.; Zou, J.; Ding, J.X.; et al. Injectable Cholesterol-Enhanced Stereocomplex Polylactide Thermogel Loading Chondrocytes for Optimized Cartilage Regeneration. Adv. Healthc. Mater. 2019, 8, 1900312. [Google Scholar] [CrossRef]
- Jia, P.; Hu, J.; Zhai, W.T.; Duan, Y.X.; Zhang, J.M.; Han, C.Y. Cell Morphology and Improved Heat Resistance of Microcellular Poly(L-lactide) Foam via Introducing Stereocomplex Crystallites of PLA. Ind. Eng. Chem. Res. 2015, 54, 2476–2488. [Google Scholar] [CrossRef]
- Di, Y.W.; Iannace, S.; Di Maio, E.; Nicolais, L. Reactively modified poly(lactic acid): Properties and foam processing. Macromol. Mater. Eng. 2005, 290, 1083–1090. [Google Scholar] [CrossRef]
- Xu, L.Q.; Huang, H.X. Foaming of Poly(lactic acid) Using Supercritical Carbon Dioxide as Foaming Agent: Influence of Crystallinity and Spherulite Size on Cell Structure and Expansion Ratio. Ind. Eng. Chem. Res. 2014, 53, 2277–2286. [Google Scholar] [CrossRef]
- Martini-Vvedensky, J.E.; Suh, N.P.; Waldman, F.A.; Massachusetts Institute of Technology. A Microcellular Closed Cell Foams and Their Method of Manufacture. U.S. Patent 4,473,665, 25 September 1984. [Google Scholar]
- Keshtkar, M.; Nofar, M.; Park, C.B.; Carreau, P.J. Extruded PLA/clay nanocomposite foams blown with supercritical CO2. Polymer 2014, 55, 4077–4090. [Google Scholar] [CrossRef]
- Ding, W.D.; Chu, R.K.M.; Mark, L.H.; Park, C.B.; Sain, M. Non-isothermal crystallization behaviors of poly(lactic acid)/cellulose nanofiber composites in the presence of CO2. Eur. Polym. J. 2015, 71, 231–247. [Google Scholar] [CrossRef]
- Yin, D.X.; Mi, J.G.; Zhou, H.F.; Wang, X.D.; Fu, H. Microcellular foaming behaviors of chain extended poly (butylene succinate)/polyhedral oligomeric silsesquioxane composite induced by isothermal crystallization. Polym. Degrad. Stabil. 2019, 167, 228–240. [Google Scholar] [CrossRef]
- Ji, G.Y.; Zhai, W.T.; Lin, D.P.; Ren, Q.; Zheng, W.G.; Jung, D.W. Microcellular Foaming of Poly(lactic acid)/Silica Nanocomposites in Compressed CO2: Critical Influence of Crystallite Size on Cell Morphology and Foam Expansion. Ind. Eng. Chem. Res. 2013, 52, 6390–6398. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D.; Li, H.B. Extrusion foaming of semi-crystalline PLA and PLA/thermoplastic starch blends. Macromol. Biosci. 2007, 7, 907–920. [Google Scholar] [CrossRef] [Green Version]
- Zhai, W.T.; Ko, Y.; Zhu, W.L.; Wong, A.S.; Park, C.B. A Study of the Crystallization, Melting, and Foaming Behaviors of Polylactic Acid in Compressed CO2. Int. J. Mol. Sci. 2009, 10, 5381–5397. [Google Scholar] [CrossRef]
- Wong, A.; Wijnands, S.F.L.; Kuboki, T.; Park, C.B. Mechanisms of nanoclay-enhanced plastic foaming processes: Effects of nanoclay intercalation and exfoliation. J. Nanopart. Res. 2013, 15, 1815. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.L.; Zhang, H.T.; Park, C.B. Continuous processing of low-density, microcellular poly(lactic acid) foams with controlled cell morphology and crystallinity. Chem. Eng. Sci. 2012, 75, 390–399. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D. Crystallinity Development in Cellular Poly(lactic acid) in the Presence of Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2009, 113, 2920–2932. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.L.; Ding, W.D.; Zhao, N.; Chen, J.B.; Park, C.B. Effects of Compressed CO2 and Cotton Fibers on the Crystallization and Foaming Behaviors of Polylactide. Ind. Eng. Chem. Res. 2018, 57, 2094–2104. [Google Scholar] [CrossRef]
- Lan, Q.F.; Li, Y. Mesophase-Mediated Crystallization of Poly(L-lactide): Deterministic Pathways to Nanostructured Morphology and Superstructure Control. Macromolecules 2016, 49, 7387–7399. [Google Scholar] [CrossRef]
- Lan, Q.F.; Li, Y.; Chi, H.T. Highly Enhanced Mesophase Formation in Glassy Poly(L-lactide) at Low Temperatures by Low-Pressure CO2 That Provides Moderately Increased Molecular Mobility. Macromolecules 2016, 49, 2262–2271. [Google Scholar] [CrossRef]
- Xu, D.W.; Zhang, H.L.; Pu, L.; Li, L. Fabrication of Poly(vinylidene fluoride)/Multiwalled carbon nanotube nanocomposite foam via supercritical fluid carbon dioxide: Synergistic enhancement of piezoelectric and mechanical properties. Compos. Sci. Technol. 2020, 192, 108108. [Google Scholar] [CrossRef]
- Li, S.J.; Chen, T.Y.; Liao, X.; Han, W.Q.; Yan, Z.H.; Li, J.S.; Li, G.X. Effect of Macromolecular Chain Movement and the Interchain Interaction on Crystalline Nucleation and Spherulite Growth of Polylactic Acid under High-Pressure CO2. Macromolecules 2020, 53, 312–322. [Google Scholar] [CrossRef]
- Fujimaki, T. Processability and properties of aliphatic polyesters, ‘BIONOLLE’, synthesized by polycondensation reaction. Polym. Degrad. Stabil. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Shi, X.T.; Wang, L.; Kang, Y.; Qin, J.B.; Li, J.T.; Zhang, H.M.; Fan, X.; Liu, Y.; Zhang, G.C. Effect of poly(butylenes succinate) on the microcellular foaming of polylactide using supercritical carbon dioxide. J. Polym. Res. 2018, 25, 229. [Google Scholar] [CrossRef]
- Jing, Z.X.; Shi, X.T.; Zhang, G.C.; Li, J.; Li, J.W.; Zhou, L.S.; Zhang, H.M. Formation, structure and promoting crystallization capacity of stereocomplex crystallite network in the poly(lactide) blends based on linear PLLA and PDLA with different structures. Polymer 2016, 92, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of Structure of Solution Grown Crystals of Lactide Copolymers by Means of Chemical-Reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Wong, A.; Park, C.B. The effects of extensional stresses on the foamability of polystyrene-talc composites blown with carbon dioxide. Chem. Eng. Sci. 2012, 75, 49–62. [Google Scholar] [CrossRef]
- Xie, L.; Xu, H.; Chen, J.B.; Zhang, Z.J.; Hsiao, B.S.; Zhong, G.J.; Chen, J.; Li, Z.M. From Nanofibrillar to Nanolaminar Poly(butylene succinate): Paving the Way to Robust Barrier and Mechanical Properties for Full-Biodegradable Poly(lactic acid) Films. ACS Appl. Mater. Interfaces 2015, 7, 8023–8032. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Sakamo, K.; Ono, Y.; Kawahara, W. Melting behavior of poly(L-lactic acid): X-ray and DSC analyses of the melting process. Polymer 2008, 49, 1943–1951. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of poly (lactic acid) (PTA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Shi, X.T.; Zhang, G.C.; Phuong, T.V.; Lazzeri, A. Synergistic Effects of Nucleating Agents and Plasticizers on the Crystallization Behavior of Poly(lactic acid). Molecules 2015, 20, 1579–1593. [Google Scholar] [CrossRef]
- Li, J.S.; Liao, X.; Yang, Q.; Li, G.X. Crystals in Situ Induced by Supercritical CO2 as Bubble Nucleation Sites on Spherulitic PLLA Foam Structure Controlling. Ind. Eng. Chem. Res. 2017, 56, 11111–11124. [Google Scholar] [CrossRef]
- Shi, X.T.; Jing, Z.X.; Zhang, G.C. Influence of PLA stereocomplex crystals and thermal treatment temperature on the rheology and crystallization behavior of asymmetric poly(L-Lactide)/poly(D-lactide) blends. J. Polym. Res. 2018, 25, 71. [Google Scholar] [CrossRef]
- Nuzzo, A.; Bilotti, E.; Peijs, T.; Aciern, D.; Filippone, G. Nanoparticle-induced co-continuity in immiscible polymer blends—A comparative study on bio-based PLA-PA11 blends filled with organoclay, sepiolite, and carbon nanotubes. Polymer 2014, 55, 4908–4919. [Google Scholar] [CrossRef]
- Yeh, J.T.; Tsou, C.H.; Huang, C.Y.; Chen, K.N.; Wu, C.S.; Chai, W.L. Compatible and Crystallization Properties of Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blends. J. Appl. Polym. Sci. 2010, 116, 680–687. [Google Scholar] [CrossRef]








| Sample Name | PLLA/wt% | PBS/wt% | PDLA/wt% | |||
|---|---|---|---|---|---|---|
| PLA/10PBS | 90 | 90% | 10 | 10% | 0 | |
| PLA/20PBS | 80 | 80% | 20 | 20% | 0 | |
| PLA/30PBS | 70 | 70% | 30 | 30% | 0 | |
| PLA/10PBS+5D | 90 | 85.7% | 10 | 9.5% | 5 | 4.8% |
| PLA/20PBS+5D | 80 | 76.2% | 20 | 19.0% | 5 | 4.8% |
| PLA/30PBS+5D | 70 | 66.7% | 30 | 28.5% | 5 | 4.5% |
| PLA/10PBS+10D | 90 | 81.8% | 10 | 9.1% | 10 | 9.1% |
| PLA/PBS +PDLA | Tg/°C | Tcc/°C | △Hcc (J/g) | Tm-PBS/°C | Tm-hc/°C | △Hm-hc (J/g) | Xm-hc (%) | Tm-sc/°C | △Hm-sc (J/g) | Xm-sc (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 90/10 | 51.1 | 85.5 | 17.9 | 111.2 | 172.3 | 43.2 | 30.0 | - | - | - |
| 80/20 | 50.8 | 86.7 | 14.5 | 113.6 | 170.1 | 37.6 | 30.9 | - | - | - |
| 70/30 | 47.1 | 80.6 | 12.2 | 110.0 | 169.9 | 33.3 | 32.1 | - | - | - |
| 90/10+5 | 53.8 | 84.5 | 19.8 | 110.1 | 171.6 | 36.0 | 19.2 | 219.6 | 9.7 | 7.5 |
| 80/20+5 | 53.2 | 83.6 | 18.7 | 110.7 | 171.3 | 34.5 | 21.0 | 213.5 | 6.6 | 5.8 |
| 70/30+5 | 51.3 | 82.7 | 14.6 | 111.1 | 171.0 | 27.2 | 19.2 | 213.0 | 4.9 | 4.9 |
| PLA/PBS | 9/1 | 8/2 | 7/3 | 9/1+5D | 8/2+5D | 7/3+5D |
|---|---|---|---|---|---|---|
| Cell Density/cm−3 | 6.36 × 109 | 2.51 × 109 | 5.04 × 109 | 2.23 × 1010 | 1.30 × 1010 | 3.82 × 109 |
| Cell Size/μm | 3.21 | 4.55 | 6.74 | 0.66 | 4.83 | 3.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wang, L.; Zhou, J.; Fan, X.; Xie, H.; Zhang, H.; Zhang, G.; Shi, X. Influence of Polylactide (PLA) Stereocomplexation on the Microstructure of PLA/PBS Blends and the Cell Morphology of Their Microcellular Foams. Polymers 2020, 12, 2362. https://doi.org/10.3390/polym12102362
Sun Z, Wang L, Zhou J, Fan X, Xie H, Zhang H, Zhang G, Shi X. Influence of Polylactide (PLA) Stereocomplexation on the Microstructure of PLA/PBS Blends and the Cell Morphology of Their Microcellular Foams. Polymers. 2020; 12(10):2362. https://doi.org/10.3390/polym12102362
Chicago/Turabian StyleSun, Zhiyuan, Long Wang, Jinyang Zhou, Xun Fan, Hanghai Xie, Han Zhang, Guangcheng Zhang, and Xuetao Shi. 2020. "Influence of Polylactide (PLA) Stereocomplexation on the Microstructure of PLA/PBS Blends and the Cell Morphology of Their Microcellular Foams" Polymers 12, no. 10: 2362. https://doi.org/10.3390/polym12102362
APA StyleSun, Z., Wang, L., Zhou, J., Fan, X., Xie, H., Zhang, H., Zhang, G., & Shi, X. (2020). Influence of Polylactide (PLA) Stereocomplexation on the Microstructure of PLA/PBS Blends and the Cell Morphology of Their Microcellular Foams. Polymers, 12(10), 2362. https://doi.org/10.3390/polym12102362