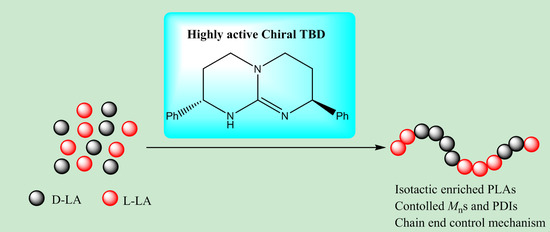
Chiral 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)-Catalyzed Stereoselective Ring-Opening Polymerization of rac-Lactide: High Reactivity for Isotactic Enriched Polylactides (PLAs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Method of rac-Lactide Polymerization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Fèvre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2017, 118, 839–885. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Tabata, Y. Polyhydroxyalkanonate derivatives in current clinical applications and trials. Adv. Drug Deliv. Rev. 2003, 55, 501–518. [Google Scholar] [CrossRef]
- Natta, G.; Pino, P.; Corradini, P.; Danusso, F.; Mantica, E.; Mazzanti, G.; Moraglio, G. Crystalline high polymers of α-olefins. J. Am. Chem. Soc. 1955, 77, 1708–1710. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Mahmood, Q.; Lv, C.; Yang, R.; Zhou, L.; Wang, Q. Asymmetric kinetic resolution polymerization. Coord. Chem. Rev. 2020, 414, 213296. [Google Scholar] [CrossRef]
- Dijkstra, P.J.; Du, H.; Feijen, J. Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym. Chem. 2011, 2, 520–527. [Google Scholar] [CrossRef]
- Pilone, A.; Press, K.; Goldberg, I.; Kol, M.; Mazzeo, M.; Lamberti, M. Gradient Isotactic Multiblock Polylactides from Aluminum Complexes of Chiral Salalen Ligands. J. Am. Chem. Soc. 2014, 136, 2940–2943. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Ishii, R.; Akakura, M.; Aoi, K. Stereoselective Ring-Opening Polymerization of Racemic Lactide Using Aluminum-Achiral Ligand Complexes: Exploration of a Chain-End Control Mechanism. J. Am. Chem. Soc. 2002, 124, 5938–5939. [Google Scholar] [CrossRef] [PubMed]
- Ottou, W.N.; Sardon, H.; Mecerreyes, D.; Vignolle, J.; Taton, D. Update and challenges in organo-mediated polymerization reactions. Prog. Polym. Sci. 2016, 56, 64–115. [Google Scholar] [CrossRef] [Green Version]
- Dove, A.P. Organic Catalysis for Ring-Opening Polymerization. ACS Macro Lett. 2012, 1, 1409–1412. [Google Scholar] [CrossRef]
- Yuan, R.; Shou, Q.; Mahmood, Q.; Xu, G.; Sun, X.; Wan, J.; Wang, Q. Kinetic Studies on Guanidine-Superbase-Promoted Ring-Opening Polymerization of ε-Caprolactone. Synlett 2019, 30, 928–931. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.R.; Breyfogle, L.E.; Hillmyer, M.A.; Tolman, W.B. Stereoelective polymerization of d,l-lactide using N-heterocyclic carbene based compounds. Chem. Commun. 2004, 10, 2504. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.P.; Li, H.; Pratt, R.C.; Lohmeijer, B.G.G.; Culkin, D.A.; Waymouth, R.M.; Hedrick, J.L. Stereoselective polymerization of rac- and meso-lactide catalyzed by sterically encumbered N-heterocyclic carbenes. Chem. Commun. 2006, 27, 2881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nederberg, F.; Messman, J.M.; Pratt, R.C.; Hedrick, J.L.; Wade, C.G. Organocatalytic Stereoselective Ring-Opening Polymerization of Lactide with Dimeric Phosphazene Bases. J. Am. Chem. Soc. 2007, 129, 12610–12611. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Zhao, N.; Li, Z. Stereoselective Ring-Opening Polymerization of rac-Lactide Using Organocatalytic Cyclic Trimeric Phosphazene Base. ACS Macro Lett. 2018, 7, 624–628. [Google Scholar] [CrossRef]
- Makiguchi, K.; Yamanaka, T.; Kakuchi, T.; Terada, M.; Satoh, T. Binaphthol-derived phosphoric acids as efficient chiral organocatalysts for the enantiomer-selective polymerization of rac-lactide. Chem. Commun. 2014, 50, 2883–2885. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, E.Y.-X. From meso-Lactide to Isotactic Polylactide: Epimerization by B/N Lewis Pairs and Kinetic Resolution by Organic Catalysts. J. Am. Chem. Soc. 2015, 137, 12506–12509. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Rivilla, I.; Agirre, M.; Basterretxea, A.; Etxeberria, A.; Veloso, A.; Sardon, H.; Mecerreyes, D.; Cossío, F.P. Enantioselective Ring-Opening Polymerization of rac-Lactide Dictated by Densely Substituted Amino Acids. J. Am. Chem. Soc. 2017, 139, 4805–4814. [Google Scholar] [CrossRef]
- Orhan, B.; Tschan, M.J.-L.; Wirotius, A.-L.; Dove, A.P.; Coulembier, O.; Taton, D. Isoselective Ring-Opening Polymerization of rac-Lactide from Chiral Takemoto’s Organocatalysts: Elucidation of Stereocontrol. ACS Macro Lett. 2018, 7, 1413–1419. [Google Scholar] [CrossRef] [Green Version]
- Lv, C.; Zhou, L.; Yuan, R.; Mahmood, Q.; Xu, G.; Wang, Q. Isoselective ring-opening polymerization and asymmetric kinetic resolution polymerization of rac-lactide catalyzed by bifunctional iminophosphorane–thiourea/urea catalysts. New J. Chem. 2020, 44, 1648–1655. [Google Scholar] [CrossRef]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Lundberg, P.N.P.; Dove, A.P.; Li, H.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L. Exploration, optimization, and application of supramolecular thiourea-amine catalysts for the synthesis of lactide (co)polymers. Macromolecules 2006, 39, 7863–7871. [Google Scholar] [CrossRef]
- Miyake, G.M.; Chen, E.Y.-X. Cinchona alkaloids as stereoselective organocatalysts for the partial kinetic resolution polymerization of rac-lactide. Macromolecules 2011, 44, 4116–4124. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Yuntawattana, N.; Beer, P.D.; Williams, C.K. Isoselective Lactide Ring Opening Polymerisation using [2] Rotaxane Catalysts. Angew. Chem. Int. Ed. 2019, 58, 6007–6011. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Waymouth, R.M.; Hedrick, J.L. Triazabicyclodecene: A simple bifunctional organocatalyst for acyl transfer and ring-opening polymerization of cyclic esters. J. Am. Chem. Soc. 2006, 128, 4556–4557. [Google Scholar] [CrossRef]
- Moins, S.; Hoyas, S.; Lemaur, V.; Orhan, B.; Chiaie, K.D.; Lazzaroni, R.; Taton, D.; Dove, A.P.; Coulembier, O. Stereoselective ROP of rac- and meso-Lactides Using Achiral TBD as Catalyst. Catalysts 2020, 10, 620. [Google Scholar] [CrossRef]
- Tidwell, T.T. Wilhelm Schlenk: The Man behind the Flask. Angew. Chem. Int. Ed. 2001, 40, 331–337. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M.; et al. Guanidine and Amidine Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Eisenreich, F.; Viehmann, P.; Müller, F.; Hecht, S. Electronic Activity Tuning of Acyclic Guanidines for Lactide Polymerization. Macromolecules 2015, 48, 8729–8732. [Google Scholar] [CrossRef]
- Kan, C.; Hu, J.; Huang, Y.; Wang, H.; Ma, H. Highly Isoselective and Active Zinc Catalysts for rac-Lactide Polymerization: Effect of Pendant Groups of Aminophenolate Ligands. Macromolecules 2017, 50, 7911–7919. [Google Scholar] [CrossRef]
- Klitzke, J.S.; Roisnel, T.; Kirillov, E.; Casagrande, O.L.; Carpentier, J. Yttrium– and Aluminum–Bis(phenolate)pyridine Complexes: Catalysts and Model Compounds of the Intermediates for the Stereoselective Ring-Opening Polymerization of Racemic Lactide and β-Butyrolactone. Organometallics 2013, 33, 309–321. [Google Scholar] [CrossRef]
- Ikpo, N.; Hoffmann, C.; Dawe, L.N.; Kerton, F.M. Ring-opening polymerization of ε-caprolactone by lithium piperazinyl-aminephenolate complexes: Synthesis, characterization and kinetic studies. Dalton Trans. 2012, 41, 6651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, S.; Thomas, A.W.; Dove, A.P. Highly Polarized Alkenes as Organocatalysts for the Polymerization of Lactones and Trimethylene Carbonate. ACS Macro Lett. 2016, 5, 134–138. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Li, Z.; Wang, H.; Gebru, H.; Chen, S.; Zhu, H.; Wei, F.; Guo, K. Organocatalyzed Anionic Ring-Opening Polymerizations of N-Sulfonyl Aziridines with Organic Superbases. ACS Macro Lett. 2017, 6, 1331–1336. [Google Scholar] [CrossRef]
- Liu, X.; Hong, M. Transesterification by air/moisture-tolerant bifunctional organocatalyst to produce ‘nonstrained’ γ-butyrolactone-based aliphatic copolyesters: Turning a bane into a boon. Eur. Polym. J. 2019, 121, 109277. [Google Scholar] [CrossRef]
- Liu, X.; Hong, M.; Falivene, L.; Cavallo, L.; Chen, E.Y.-X. Closed-Loop Polymer Upcycling by Installing Property-Enhancing Comonomer Sequences and Recyclability. Macromolecules 2019, 52, 4570–4578. [Google Scholar] [CrossRef]
- Petrus, R.; Bykowski, D.; Sobota, P. Solvothermal Alcoholysis Routes for Recycling Polylactide Waste as Lactic Acid Esters. ACS Catal. 2016, 6, 5222–5235. [Google Scholar] [CrossRef]
- Whitelaw, E.L.; Davidson, M.G.; Jones, M.D. Group 4 salalen complexes for the production and degradation of polylactide. Chem. Commun. 2011, 47, 10004–10006. [Google Scholar] [CrossRef] [Green Version]
- Mou, Z.; Liu, B.; Wang, M.; Xie, H.; Li, P.; Li, L.; Li, S.; Cui, D. Isoselective ring-opening polymerization of rac-lactide initiated by achiral heteroscorpionate zwitterionic zinc complexes. Chem. Commun. 2014, 50, 11411–11414. [Google Scholar] [CrossRef]






| Run | rac-LA/Cat./BnOH | Temp. (°C) | Time (min) | Conv. (%) b | Mnc (g/mol) | Mw/Mnc | Pmd |
|---|---|---|---|---|---|---|---|
| 1 | 100/1/1 | 25 | 1 | >99 | 15,700 | 1.30 | 0.67 |
| 2 | 100/1/2 | 25 | 1 | >99 | 10,900 | 1.47 | 0.61 |
| 3 | 100/1/0 | 25 | 30 | >99 | 17,400 | 1.75 | 0.64 |
| 4 | 100/1/1 | 0 | 60 | >99 | 19,700 | 1.62 | 0.69 |
| 5 e | 100/1/1 | 25 | 30 | 95 | 12,100 | 2.07 | 0.52 |
| 6 f | 100/1/1 | 25 | 60 | >99 | 15,500 | 2.14 | 0.62 |
| 7 | 200/1/1 | 25 | 5 | >99 | 29,400 | 1.39 | 0.67 |
| 8 | 300/1/1 | 25 | 30 | >99 | 38,200 | 1.32 | 0.66 |
| 9 | 400/1/1 | 25 | 60 | >99 | 48,800 | 1.29 | 0.68 |
| 10 | 500/1/1 | 25 | 15 | 29 | 26,300 | 1.30 | 0.64 |
| 11 | 500/1/1 | 25 | 30 | 52 | 39,500 | 1.28 | 0.62 |
| 12 | 500/1/1 | 25 | 45 | 63 | 47,400 | 1.28 | 0.65 |
| 13 | 500/1/1 | 25 | 60 | 95 | 57,400 | 1.23 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, Q.; Xu, G.; Zhou, L.; Guo, X.; Wang, Q. Chiral 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)-Catalyzed Stereoselective Ring-Opening Polymerization of rac-Lactide: High Reactivity for Isotactic Enriched Polylactides (PLAs). Polymers 2020, 12, 2365. https://doi.org/10.3390/polym12102365
Mahmood Q, Xu G, Zhou L, Guo X, Wang Q. Chiral 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)-Catalyzed Stereoselective Ring-Opening Polymerization of rac-Lactide: High Reactivity for Isotactic Enriched Polylactides (PLAs). Polymers. 2020; 12(10):2365. https://doi.org/10.3390/polym12102365
Chicago/Turabian StyleMahmood, Qaiser, Guangqiang Xu, Li Zhou, Xuanhua Guo, and Qinggang Wang. 2020. "Chiral 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)-Catalyzed Stereoselective Ring-Opening Polymerization of rac-Lactide: High Reactivity for Isotactic Enriched Polylactides (PLAs)" Polymers 12, no. 10: 2365. https://doi.org/10.3390/polym12102365
APA StyleMahmood, Q., Xu, G., Zhou, L., Guo, X., & Wang, Q. (2020). Chiral 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)-Catalyzed Stereoselective Ring-Opening Polymerization of rac-Lactide: High Reactivity for Isotactic Enriched Polylactides (PLAs). Polymers, 12(10), 2365. https://doi.org/10.3390/polym12102365