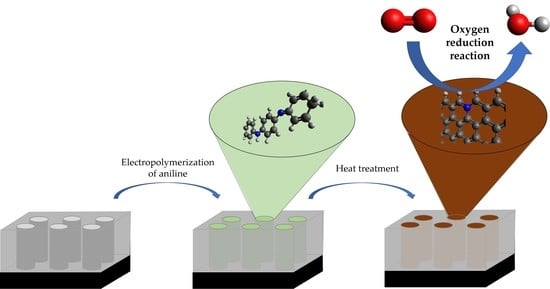
Polyaniline-Derived N-Doped Ordered Mesoporous Carbon Thin Films: Efficient Catalysts towards Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Silica Template
2.3. Titania Template
2.4. PANI Electropolymerization
2.5. Heat Treatments
2.6. Physicochemical Characterization
2.7. Electrochemical Characterization
3. Results and Discussion
3.1. Electrochemical Polymerization and Characterization of G/Si-PANI and G/Ti-PANI Composites
3.2. Electrochemical Characterization of Ordered Mesoporous N-Doped Carbon-Based Composites
3.3. Physicochemical Characterization
3.4. Electrocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The recent progress and future of oxygen reduction reaction catalysis: A review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Shahgaldi, S.; Hamelin, J. Improved carbon nanostructures as a novel catalyst support in the cathode side of PEMFC: A critical review. Carbon 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Lv, H.; Li, D.; Strmcnik, D.; Paulikas, A.P.; Markovic, N.M.; Stamenkovic, V.R. Recent advances in the design of tailored nanomaterials for efficient oxygen reduction reaction. Nano Energy 2016, 29, 149–165. [Google Scholar] [CrossRef]
- Wu, Z.; Iqbal, Z.; Wang, X. Metal-free, carbon-based catalysts for oxygen reduction reactions. Front. Chem. Sci. Eng. 2015, 9, 280–294. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, L. Heteroatom-Doped Graphitic Carbon Catalysts for Efficient Electrocatalysis of Oxygen Reduction Reaction. ACS Catal. 2015, 5, 7244–7253. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Dai, L. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, e1500564. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Zhao, S.; Lu, X.; Wang, L.; Gale, J.; Amal, R. Carbon-Based Metal-Free Catalysts for Electrocatalytic Reduction of Nitrogen for Synthesis of Ammonia at Ambient Conditions. Adv. Mater. 2019, 31, 1805367. [Google Scholar] [CrossRef]
- Wu, Z.; Song, M.; Wang, J.; Liu, X. Recent Progress in Nitrogen-Doped Metal-Free Electrocatalysts for Oxygen Reduction Reaction. Catalysts 2018, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Yu, J.; Huang, T.; Sun, M. Recent Advance on Polyaniline or Polypyrrole-Derived Electrocatalysts for Oxygen Reduction Reaction. Polymers 2018, 10, 1397. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef]
- Wang, L.; Jia, W.; Liu, X.; Li, J.; Titirici, M.M. Sulphur-doped ordered mesoporous carbon with enhanced electrocatalytic activity for the oxygen reduction reaction. J. Energy Chem. 2016, 25, 566–570. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Dou, S.; Shen, A.; Tao, L.; Dai, L.; Wang, S. Sulfur-doped graphene derived from cycled lithium-sulfur batteries as a metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2015, 54, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Quílez-Bermejo, J.; González-Gaitán, C.; Morallón, E.; Cazorla-Amorós, D. Effect of carbonization conditions of polyaniline on its catalytic activity towards ORR. Some insights about the nature of the active sites. Carbon 2017, 119, 62–71. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, G.; Huang, F.; Zhang, Y.; Pan, M. Polyaniline derived N- and O-enriched high surface area hierarchical porous carbons as an efficient metal-free electrocatalyst for oxygen reduction. Electrochim. Acta 2017, 257, 73–81. [Google Scholar] [CrossRef]
- Silva, R.; Voiry, D.; Chhowalla, M.; Asefa, T. Efficient metal-free electrocatalysts for oxygen reduction: Polyaniline-derived N- and O-doped mesoporous carbons. J. Am. Chem. Soc. 2013, 135, 7823–7826. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.W.; Wei, W.; Wu, Z.S.; Feng, X.; Müllen, K. Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [Green Version]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-Free Heteroatom-doped Carbon-based catalysts for ORR. A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Sharifi, T.; Hu, G.; Jia, X.; Wågberg, T. Formation of Active Sites for Oxygen Reduction Reactions by Transformation of Nitrogen Functionalities in Nitrogen-Doped Carbon Nanotubes. ACS Nano 2012, 6, 8904–8912. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Oxygen-reduction catalysis of N-doped carbons prepared: Via heat treatment of polyaniline at over 1100 °C. Chem. Commun. 2018, 54, 4441–4444. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Hou, Z.; Chai, G.L.; Terakura, K. Possible oxygen reduction reactions for graphene edges from first principles. J. Phys. Chem. C 2014, 118, 17616–17625. [Google Scholar] [CrossRef]
- Ikeda, T.; Boero, M.; Huang, S.F.; Terakura, K.; Oshima, M.; Ozaki, J. Carbon alloy catalysts: Active sites for oxygen reduction reaction. J. Phys. Chem. C 2008, 112, 14706–14709. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Nitrogen doped superporous carbon prepared by a mild method. Enhancement of supercapacitor performance. Int. J. Hydrogen Energy 2016, 41, 19691–19701. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Pašti, I.; Gavrilov, N.; Janošević, A.; Mentus, S. Carbonised polyaniline and polypyrrole: Towards advanced nitrogen-containing carbon materials. Chem. Pap. 2013, 67, 781–813. [Google Scholar] [CrossRef]
- Lee, U.H.; Yang, J.H.; Lee, H.J.; Park, J.Y.; Lee, K.R.; Kwon, Y.U. Facile and adaptable synthesis method of mesostructured silica thin films. J. Mater. Chem. 2008, 18, 1881–1888. [Google Scholar] [CrossRef]
- Leyva-García, S.; Lozano-Castelló, D.; Morallón, E.; Cazorla-Amorós, D. Silica-templated ordered mesoporous carbon thin films as electrodes for micro-capacitors. J. Mater. Chem. A 2016, 4, 4570–4579. [Google Scholar] [CrossRef] [Green Version]
- Oveisi, H.; Jiang, X.; Nemoto, Y.; Beitollahi, A.; Yamauchi, Y. Cerium-doped mesoporous TiO2 thin films: Controlled crystallization of anatase with retention of highly ordered mesostructure. Microporous Mesoporous Mater. 2011, 139, 38–44. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Berenguer-Murcia, Á.; Cabello, M.; Cazorla-Amorós, D.; Tirado, J.L. Ordered mesoporous titanium oxide for thin film microbatteries with enhanced lithium storage. Electrochim. Acta 2015, 166, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Bleda-Martínez, M.J.; Morallón, E.; Cazorla-Amorós, D. Polyaniline/porous carbon electrodes by chemical polymerisation: Effect of carbon surface chemistry. Electrochim. Acta 2007, 52, 4962–4968. [Google Scholar] [CrossRef] [Green Version]
- Montilla, F.; Cotarelo, M.Á.; Morallón, E. Hybrid sol-gel-conducting polymer synthesised by electrochemical insertion: Tailoring the capacitance of polyaniline. J. Mater. Chem. 2009, 19, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.C.; Lin, J.Y. Effects of the loading and polymerization temperature on the capacitive performance of polyaniline in NaNO3. Electrochim. Acta 2002, 47, 4055–4067. [Google Scholar] [CrossRef]
- Planes, G.A.; Rodríguez, J.L.; Miras, M.C.; García, G.; Pastor, E.; Barbero, C.A. Spectroscopy evidence for intermediate species formed during aniline polymerization and polyaniline degradation. Phys. Chem. Chem. Phys. 2010, 12, 10584–10593. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Ayupov, A.B.; Yeletsky, P.M.; Lebedeva, M.V. Influence of monomer content on course of aniline polymerization in presence of high surface area carbon. J. Electroanal. Chem. 2019, 835, 73–80. [Google Scholar] [CrossRef]
- Duic, L.; Mandic, Z.; Kovac, S. Polymer-dimer distribution in the electrochemical synthesis of polyaniline. Electrochim. Acta 1995, 40, 1681–1688. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Cazorla-Amorós, D.; Berenguer-Murcia, Á. Facile encapsulation of P25 (TiO2) in spherical silica with hierarchical porosity with enhanced photocatalytic properties for gas-phase propene oxidation. Appl. Catal. A Gen. 2018, 564, 123–132. [Google Scholar]
- Fröschl, T.; Hörmann, U.; Kubiak, P.; Kučerová, G.; Pfanzelt, M.; Weiss, C.K.; Behm, R.J.; Hüsing, N.; Kaiser, U.; Landfester, K.; et al. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Shao, Y.; Zhang, L.; Li, Y.; Wu, Y.; Hao, X. Transition-Metal Oxynitride: A Facile Strategy for Improving Electrochemical Capacitor Storage. Adv. Mater. 2019, 31, 1806088. [Google Scholar] [CrossRef] [PubMed]
- Arias-Pardilla, J.; Salavagione, H.J.; Barbero, C.; Morallón, E.; Vázquez, J.L. Study of the chemical copolymerization of 2-aminoterephthalic acid and aniline. Synthesis and copolymer properties. Eur. Polym. J. 2006, 42, 1521–1532. [Google Scholar] [CrossRef]
- Rannou, P.; Rouchon, D.; Nicolau, Y.F.; Nechtschein, M.; Ermolieff, A. Chemical degradation of aged CSA-protonated PANI films analyzed by XPS. Synth. Met. 1999, 101, 823–824. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Ghisolfi, A.; Grau-Marín, D.; San-Fabián, E.; Morallón, E.; Cazorla-Amorós, D. Post-synthetic efficient functionalization of polyaniline with phosphorus-containing groups. Effect of phosphorus on electrochemical properties. Eur. Polym. J. 2019, 119, 272–280. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Yin, L.C.; Lu, X.; Chen, Z.G.; Gentle, I.R.; Lu, G.Q.; Cheng, H.M. Nitrogen-doped carbon monolith for alkaline supercapacitors and understanding nitrogen-induced redox transitions. Chem. A Eur. J. 2012, 18, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Falco, C.; Sevilla, M.; White, R.J.; Rothe, R.; Titirici, M.M. Renewable nitrogen-doped hydrothermal carbons derived from microalgae. ChemSusChem 2012, 5, 1834–1840. [Google Scholar] [CrossRef] [Green Version]
- Streubel, P.; Hesse, R.; Friebel, J.; Schmiers, H.; Köpsel, R. Change of chemical bonding of nitrogen of polymeric N-heterocyclic compounds during pyrolysis. Carbon 2002, 37, 1965–1978. [Google Scholar]
- Lahaye, J.; Nansé, G.; Bagreev, A.; Strelko, V. Porous structure and surface chemistry of nitrogen containing carbons from polymers. Carbon 1999, 37, 585–590. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, X.; Li, M.; Wang, F.; Wang, Q.; Kang, G.; Peng, F. Novel silicon-doped, silicon and nitrogen-codoped carbon nanomaterials with high activity for the oxygen reduction reaction in alkaline medium. J. Mater. Chem. A 2015, 3, 3289–3293. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.C.; Liu, Y.J.; Zhao, J.X.; Cai, Q.H.; Wang, X.Z. Can Si-doped graphene activate or dissociate O2 molecule? J. Mol. Graph. Model. 2013, 39, 126–132. [Google Scholar] [CrossRef]
- Kim, J.H.; Ishihara, A.; Mitsushima, S.; Kamiya, N.; Ota, K.I. Catalytic activity of titanium oxide for oxygen reduction reaction as a non-platinum catalyst for PEFC. Electrochim. Acta 2007, 52, 2492–2497. [Google Scholar] [CrossRef]
- Yeager, E. Electrocatalysts for O2 reduction. Electrochim. Acta 1984, 29, 1527–1537. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, Á.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Zhang, T.; Ge, J.; Yin, Y. Permeable silica shell through surface-protected etching. Nano Lett. 2008, 8, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Quílez-Bermejo, J.; Melle-Franco, M.; San-Fabián, E.; Morallón, E.; Cazorla-Amorós, D. Towards understanding the active sites for the ORR in N-doped carbon materials through fine-tuning of nitrogen functionalities: An experimental and computational approach. J. Mater. Chem. A 2019, 7, 24239–24250. [Google Scholar] [CrossRef] [Green Version]







| Electrode | Time | Q/mC | Polyaniline Amount */µg |
|---|---|---|---|
| G_PANI | 10 s | 2.64 | 2 |
| 30 s | 7.43 | 7 | |
| 5 min | 18.25 | 17 | |
| G/Si_PANI | 10 s | 13.80 | 13 |
| 30 s | 30.39 | 29 | |
| 5 min | 70.92 | 67 | |
| G/Ti_PANI | 10 s | 19.83 | 19 |
| 30 s | 38.31 | 36 | |
| 5 min | 93.95 | 88 |
| Sample | N+/Ntotal Ratio | N Content/at.% |
|---|---|---|
| G/Si_PANI_10s | 0.39 | 0.3 |
| G/Si_PANI_30 s | 0.49 | 0.6 |
| G/Si_PANI_5 min | 0.64 | 0.7 |
| G/Ti_PANI_10s | 0.25 | 0.6 |
| G/Ti_PANI_30 s | 0.36 | 0.8 |
| G/Ti_PANI_5 min | 0.42 | 1.2 |
| G/Si_PANI_10 s_900 | - | <0.2 |
| G/SI_PANI_30 s_900 | - | 0.2 |
| G/Si_PANI_5 min_900 | - | 0.4 |
| G/Ti_PANI_10 s_900 | - | <0.2 |
| G/Ti_PANI_30 s_900 | - | 0.3 |
| G/Ti_PANI_5 min_900 | - | 0.5 |
| Sample | EONSET/V vs. RHE (−0.05 mA·cm−2) | Tafel Slope/mV·dec−1 |
|---|---|---|
| G | 0.80 | 73 |
| G/Si | 0.83 | 65 |
| G/Si_900 | 0.87 | 77 |
| G/Si_PANI_10 s_900 | 0.87 | 80 |
| G/Si_PANI_30 s_900 | 0.92 | 89 |
| G/Si_PANI_5 min_900 | 0.91 | 80 |
| G/Ti | 0.81 | 84 |
| G/Ti_900 | 0.84 | 110 |
| G/Ti_PANI_10 s_900 | 0.84 | 131 |
| G/Ti_PANI_30 s_900 | 0.89 | 118 |
| G/Ti_PANI_5 min_900 | 0.87 | 124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Polyaniline-Derived N-Doped Ordered Mesoporous Carbon Thin Films: Efficient Catalysts towards Oxygen Reduction Reaction. Polymers 2020, 12, 2382. https://doi.org/10.3390/polym12102382
Quílez-Bermejo J, Morallón E, Cazorla-Amorós D. Polyaniline-Derived N-Doped Ordered Mesoporous Carbon Thin Films: Efficient Catalysts towards Oxygen Reduction Reaction. Polymers. 2020; 12(10):2382. https://doi.org/10.3390/polym12102382
Chicago/Turabian StyleQuílez-Bermejo, Javier, Emilia Morallón, and Diego Cazorla-Amorós. 2020. "Polyaniline-Derived N-Doped Ordered Mesoporous Carbon Thin Films: Efficient Catalysts towards Oxygen Reduction Reaction" Polymers 12, no. 10: 2382. https://doi.org/10.3390/polym12102382
APA StyleQuílez-Bermejo, J., Morallón, E., & Cazorla-Amorós, D. (2020). Polyaniline-Derived N-Doped Ordered Mesoporous Carbon Thin Films: Efficient Catalysts towards Oxygen Reduction Reaction. Polymers, 12(10), 2382. https://doi.org/10.3390/polym12102382