Polymeric Ionic Liquids Derived from L-Valine for the Preparation of Highly Selective Silica-Supported Stationary Phases in Gas Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Materials, and Instrumentation
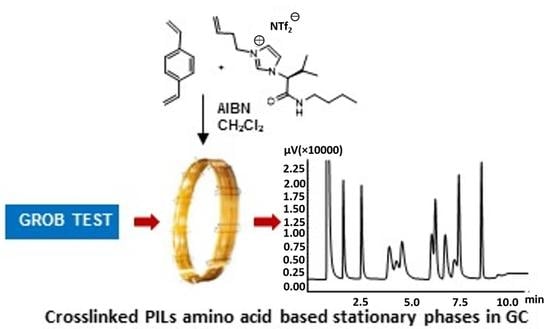
2.2. Chemical Synthesis
2.2.1. Synthesis of (S)-3-(but-3-en-1-yl)-1-(1-(butylamino)-3-methyl-1-oxobutan-2-yl)-1H-imidazol-3-ium bromide 3
2.2.2. Synthesis of (S)-3-(but-3-en-1-yl)-1-(1-(butylamino)-3-methyl-1-oxobutan-2-yl)-1H-imidazol-3-ium triflamide 4
2.3. Column Preparation
3. Results and Discussion
3.1. Optimization of Film Thickness Capillary Columns
3.2. Thermal Stability
3.3. Comparison of Interaction Parameters of PILs-Based Stationary Phases
3.4. Chromatographic Performance of the Mono and Cross-Linked Ionic Liquid Stationary Phase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Leitner, W.; Jessop, P.G.; Li, C.-J.; Wasserscheid, P.; Stark, A. Handbook of Green Chemistry—Green Solvents; Wiley-VCH Verlag GmbH: New York, NY, USA, 2010. [Google Scholar]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200. [Google Scholar] [CrossRef]
- Montolio, S.; Altava, B.; García-Verdugo, E.; Luis, S.V. Supported ILs/Materials based on ILs for developing Green Synthetic Processes and Procedures. In Green Synthetic Processes and Procedures; Ballini, R., Ed.; Green Chemistry Series No. 61; Royal Society of Chemistry: Cambridge, UK, 2019; Chapter 13; pp. 289–318. [Google Scholar]
- Yuan, J.Y.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, S.; Sun, J.-K.; Antonietti, M.; Yuan, J. Poly(ionic liquid)s with engineered nanopores for energy and environmental applications. Polymer 2020, 202, 122640. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 36, 6435–6451. [Google Scholar] [CrossRef] [Green Version]
- Morinaga, T.; Honma, S.; Ishizuka, T.; Kamijo, T.; Sato, T.; Tsujii, Y. Synthesis of Monodisperse Silica Particles Grafted with Concentrated Ionic Liquid-Type Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization for Use as a Solid State Polymer Electrolyte. Polymers 2016, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Liang, Y.N.; Hu, X. Thermally responsive ionic liquids and polymeric ionic liquids:emergingtrends and possibilities. Curr. Opin. Chem. Eng. 2019, 25, 43–50. [Google Scholar] [CrossRef]
- García-Verdugo, E.; Altava, B.; Burguete, M.I.; Lozano, P.; Luis, S.V. Ionic liquids and continuous flow processes: A good marriage to design sustainable processes. Green Chem. 2015, 17, 2693–2713. [Google Scholar] [CrossRef] [Green Version]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; Del Castillo, L.F.; Compan, V. Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Proton Exchange Membrane (PEM) Fuel Cell Applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Villa, R.; Alvarez, E.; Porcar, R.; Garcia-Verdugo, E.; Luis, S.V.; Lozano, P. Ionic liquids as an enabling tool to integrate reaction and separation processes. Green Chem. 2019, 21, 6527–6544. [Google Scholar] [CrossRef]
- Patinha, D.J.S.; Wang, H.; Yuan, J.; Rocha, S.M.; Silvestre, A.J.D.; Marrucho, I.M. Thin Porous Poly(ionic liquid) Coatings for Enhanced Headspace Solid Phase Microextraction. Polymers 2020, 12, 1909. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Feng, X.L.; Zhang, Y.P.; Yu, Q.; Wang, X.H.; Tian, M.K. Determination of Volatile Water Pollutants Using Cross-Linked Polymeric Ionic Liquid as Solid Phase Micro-Extraction Coatings. Polymers 2020, 12, 292. [Google Scholar] [CrossRef] [Green Version]
- Nan, H.; Anderson, J.L. Ionic liquid stationary phases for multidimensional gas chromatography. Trends Anal. Chem. 2018, 105, 367–379. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of ionic liquids in analytical chemistry. Anal Chem. 2019, 91, 505–531. [Google Scholar] [CrossRef] [PubMed]
- Gónzalez-Álvarez, J.; Blanco-Gomis, D.; Arias-Abrodo, P.; Díaz-Llorente, D.; Busto, E.; Ríos-Lombardía, N.; Gotor-Fernández, V.; Gutierrez-Álvarez, M.D. Polymeric imidazolium ionic liquids as valuable stationary phases in gas chromatography: Chemical synthesis and full characterization. Anal. Chim. Acta 2012, 721, 173–181. [Google Scholar]
- Poole, C.F.; Poole, S.W. Ionic liquid stationary phases for gas chromatography. J. Sep. Sci. 2011, 34, 880–900. [Google Scholar] [CrossRef]
- Cagliero, C.; Mazzucotelli, M.; Rubiolo, P.; Marengo, A.; Galli, S.; Anderson, J.L.; Sgorbini, B.; Bicchi, C. Can the selectivity of phosphonium based ionic liquids be exploited as stationary phase for routine gas chromatography? A case study: The use of trihexyl(tetradecyl) phosphonium chloride in the flavor, fragrance and natural product fields. J. Chromatogr. A 2020, 1619, 460969. [Google Scholar]
- Twu, P.; Zhao, Q.; Pitner, W.R.; Acree, W.E.; Baker, G.A.; Anderson, J.L. Evaluating the solvation properties of functionalized ionic liquids with varied cation/anion composition using the solvation parameter model. J. Chromatogr. A 2011, 1218, 5311–5338. [Google Scholar] [CrossRef]
- Ragonese, C.; Sciarrone, D.; Tranchida, P.Q.; Dugo, P.; Mondello, L. Use of ionic liquids as stationary phases in hyphenated gas chromatography techniques. J. Chromatogr. A 2012, 1255, 130–144. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Galindo-Iranzo, P.; Soria, A.C.; Sanz, M.L.; Quintanilla-López, J.E.; Lebrón-Aguilar, R. Characterization by the solvation parameter model of the retention properties of commercial ionic liquid columns for gas chromatography. J. Chromatogr. A 2014, 1326, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Shashkov, M.V.; Sidelnikov, V.N. Orthogonality and Quality of GC×GC Separations for Complex Samples with Ionic Liquid Stationary Phases in First Dimension. Chromatographia 2019, 82, 615–624. [Google Scholar] [CrossRef]
- Berthod, A.; Ruíz-Ángel, M.; Carda-Broch, S. Ionic liquids in separation techniques. J. Chromatogr. A 2008, 1184, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Han, X.; Zhang, X.; Armstrong, D.W. PEG-linked germinal dicationic ionic liquids as selective, high-stability gas chromatography stationary phases. Anal. Bioanal. Chem. 2007, 389, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Patil, R.A.; Sidisky, L.M.; Berthod, A.; Armstrong, D.W. Variation of anionic moieties of dicationic ionic liquid GC stationary phases: Effect on stability and selectivity. Anal. Chim. Acta 2018, 1042, 155e164. [Google Scholar] [CrossRef]
- Giernoth, R. Task-specific ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef]
- Fukumoto, K.M.; Yoshiziwa, M.; Ohno, H. Room temperature ionic liquids from 20 natural aminoacids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Altava, B.; Barbosa, D.S.; Burguete, M.I.; Escorihuela, J.; Luis, S.V. Synthesis of new chiral imidazolium salts derived from amino acids: Their evaluation in chiral molecular recognition. Tetrahedron Asymm. 2009, 20, 999–1003. [Google Scholar] [CrossRef]
- Gonzalez, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Luis, S.V. Chiral Room Temperature Ionic Liquids as Enantioselective Promoters for the Asymmetric Aldol Reaction. Eur. J. Org. Chem. 2014, 24, 5356–5363. [Google Scholar] [CrossRef]
- Ossowicz, P.; Klebeko, J.; Roman, R.; Janus, E.; Rozwadowski, Z. The Relationship between the Structure and Properties of Amino Acid Ionic Liquids. Molecules 2019, 24, 3252. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, L.; Altava, B.; Burguete, M.I.; Escorihuela, J.; Hernando, E.; Luis, S.V.; Quesada, R.; Vicent, C. Bis(imidazolium) salts derived from amino acids as receptors and transport agents for chloride anions. RSC Adv. 2015, 5, 34415–34423. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Luis, S.V.; Quesada, R.; Vicent, C. Application of optically active chiral bis-(imidazolium) salts as potential receptors of chiraldicarboxylate salts of biological relevance. Org. Biomol. Chem. 2015, 13, 5450–5459. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Alvarez, J.G.; Fernandez, C.; Arias, P.; Altava, B.; Burguete, M.I.; Luis, S.V.; Gutiérrez, M.D. Gas Chromatographic Analysis of Fatty Acid Methyl Esters of Milk Fat by an Ionic Liquid Derived From L-Phenylalanine as the Stationary Phase. Talanta 2015, 143, 212–218. [Google Scholar]
- Poole, C.F.; Lenca, N. Gas chromatography on wall-coated open-tubular columns with ionic liquid stationary phases. J. Chromatogr. A 2014, 1357, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Odugbesi, G.A.; Nana, H.; Soltani, M.; Davis, J.H., Jr.; Anderson, J.L. Ultra-high thermal stability perarylated ionic liquids as gas chromatographic stationary phases for the selective separation of polyaromatic hydrocarbons and polychlorinated biphenyls. J. Chromatogr. A 2019, 1604, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Cagliero, C.; Bicchi, C. Ionic liquids as gas chromatographic stationary phases: How can they change food and natural product analyses? Anal. Bioanal. Chem. 2020, 412, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Roeleveld, K.; David, F.; Lynen, F. Comparison between polymerized ionic liquids synthesized using chain-growth and step-growth mechanisms used as stationary phase in gas chromatography. J. Chromatogr. A 2016, 1451, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Anderson, J.L. Highly selective GC stationary phases consisting of binary mixtures of polymeric ionic liquids. J. Sep. Sci. 2010, 33, 79–87. [Google Scholar] [CrossRef]
- Ho, W.-Y.; Hsieh, Y.-N.; Lin, W.-C.; Kao, C.L.; Huang, P.-C.; Yeh, C.-F.; Pane, C.-Y.; Kuei, C.-H. High temperature imidazolium ionic polymer for gas chromatography. Anal. Methods 2010, 2, 455–457. [Google Scholar] [CrossRef]
- González-Álvarez, J.; Arias-Abrodo, P.; Puerto, M.; Viguri, M.E.; Pérez, J.; Gutiérrez-Álvarez, M.D. Polymerized phosphonium-based ionic liquids as stationary phases in gas chromatography: Performance improvements by addition of graphene oxide. New J. Chem. 2015, 39, 8560–8568. [Google Scholar]
- Anderson, J.L.; Armstrong, D.W. Immobilized ionic liquids as high-selectivity/high- temperature/high-stability gas chromatography stationary phases. Anal. Chem. 2005, 77, 6453–6462. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ingram, I.C.; Hantao, L.W.; Anderson, J.L. Identifying important structural features of ionic liquid stationary phases for the selective separation of nonpolar analytes by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2015, 1386, 89–97. [Google Scholar] [CrossRef]
- Zhang, C.; Park, R.A.; Anderson, J.L. Crosslinked structurally-tuned polymeric ionic liquids as stationary phases for the analysis of hydrocarbons in kerosene and diesel fuels by comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2016, 1440, 160–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, L.; Altava, B.; Bolte, M.; Burguete, M.I.; García-Verdugo, E.; Luis, S.V. Synthesis of Chiral Room Temperature Ionic Liquids (RTCILs) derived from Amino Acids. Application in Chiral Molecular Recognition. Eur. J. Org. Chem. 2012, 4996–5009. [Google Scholar] [CrossRef] [Green Version]
- Vitha, M.; Carr, P.W. The chemical interpretation and practice of linear solvation energy relationships in chromatography. J. Chromatogr. A 2006, 1126, 143–194. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Poole, C.F.; Poole, S.K. Classification of stationary phases and other material by gas chromatography. J. Chromatogr. A 1999, 842, 79–114. [Google Scholar] [CrossRef]
- Baltazar, Q.Q.; Leininger, S.K.; Anderson, J.L. Binary ionic liquids mixtures as gas chromatography stationary phases for improving the separation of selectivity of alcohols and aromatic compounds. J. Chromatogr. A 2008, 1182, 119–127. [Google Scholar] [CrossRef]
- Altava, B.; Burguete, M.I.; García-Verdugo, E.; Luis, S.V.; Vicent, M.J.; Mayoral, J.A. Supported Chiral Catalysts: The Role of the Polymeric Network. React. Funct. Polym. 2001, 48, 25–35. [Google Scholar] [CrossRef]
- Altava, B.; Burguete, M.I.; Garcia-Verdugo, E.; Luis, S.V. Chiral catalysts immobilized on achiral polymers: Effect of the polymer support on the performance of the catalyst. Chem. Soc. Rev. 2018, 47, 2722–2771. [Google Scholar] [CrossRef] [Green Version]
- Nan, H.; Zhang, C.; O’Brien, R.A.; Benchea, A.; Davis, J.H.; Anderson, J.L. Lipidic ionic liquid stationary phases for the separation of aliphatic hydrocarbons by comprehensive two-dimensional gas chromathography. J. Chromatogr. A 2017, 1481, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.L.; Armstrong, D.W. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal. Chem. 2003, 75, 4851–4858. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.L.; Ding, J.; Welthon, T.; Armstrong, D.W. Characterizing ionic liquids on the bases of multiple solvation interaction. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [Green Version]
- Shashkov, M.V.; Sidelnikov, V.N. Properties of columns with several pydridinium and imidazolium ionic liquid stationary phases. J. Chromatogr. A 2013, 1309, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Grob, K., Jr.; Grob, G.; Grob, K. Comprehensive standarized quality test for glass capillary columns. J. Chromatogr. 1978, 156, 1–20. [Google Scholar] [CrossRef]
- Grob, K.; Grob, G.; Grob, K., Jr. Testing capillary gas chromatography columns. J. Chromatogr. 1981, 219, 13–20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |





| Probe Molecules | E | S | A | B | L |
|---|---|---|---|---|---|
| 1-Hexanol | 0.21 | 0.44 | 0.344 | 0.52 | 3.643 |
| Anisol | 0.712 | 0.768 | 0 | 0.311 | 3.808 |
| Cyclohexanone | 0.403 | 0.895 | 0 | 0.53 | 3.759 |
| 1,2-Dichlorobenzene | 0.872 | 0.771 | 0 | 0.054 | 4.516 |
| Butyl benzene | 0.595 | 0.499 | 0 | 0.139 | 4.734 |
| 1-Nonanal | 0.121 | 0.636 | 0 | 0.414 | 4.838 |
| Butyl Acetate | 0.079 | 0.57 | 0 | 0.438 | 3.409 |
| Iodobenceno | 1.182 | 0.784 | 0 | 0.135 | 4.548 |
| 1-Nitropropane | 0.243 | 0.925 | 0.049 | 0.27 | 2.878 |
| 1-Pentanol | 0.219 | 0.44 | 0.344 | 0.52 | 3.128 |
| Pyridine | 0.635 | 0.843 | 0 | 0.532 | 3.006 |
| Benzonitrile | 0.742 | 1.135 | 0 | 0.331 | 4.04 |
| Nitrobenzene | 0.846 | 1.138 | 0 | 0.269 | 4.539 |
| Naphthalene | 1.24 | 0.906 | 0 | 0.193 | 5.154 |
| 2-Heptanone | 0.123 | 0.662 | 0 | 0.496 | 3.781 |
| 1-Phenyl ethanol | 0.823 | 0.819 | 0.351 | 0.648 | 4.424 |
| Benzaldehyde | 0.813 | 1.025 | 0 | 0.394 | 4.005 |
| 1-Octanol | 0.199 | 0.44 | 0.344 | 0.52 | 4.648 |
| 1-Chloronaphthalene | 1.419 | 0.951 | 0 | 0.135 | 6.175 |
| Aniline | 0.955 | 1.003 | 0.249 | 0.425 | 3.956 |
| Fluorene | 1.664 | 1.12 | 0 | 0.252 | 6.921 |
| 4-Chloroaniline | 1.017 | 1.128 | 0.366 | 0.309 | 4.972 |
| Benzyl Alcohol | 0.803 | 0.882 | 0.4 | 0.557 | 4.244 |
| Cynammyl Alcohol | 1.119 | 0.971 | 0.451 | 0.606 | 5.475 |
| Phenol | 0.769 | 0.759 | 0.716 | 0.319 | 3.844 |
| Acetophenone | 0.806 | 1.026 | 0 | 0.503 | 4.533 |
| 3-Methyl-1-butanol | 0.198 | 0.423 | 0.351 | 0.501 | 2.963 |
| 1-Butanol | 0.224 | 0.44 | 0.344 | 0.52 | 2.578 |
| Ethyl Benzoate | 0.694 | 0.886 | 0 | 0.444 | 5.032 |
| 2-Phenyl ethanol | 0.787 | 0.797 | 0.39 | 0.636 | 4.741 |
| 1-Decanol | 0.191 | 0.44 | 0.344 | 0.52 | 5.589 |
| Undecane | 0 | 0 | 0 | 0 | 5.185 |
| Styrene | 0.849 | 0.671 | 0 | 0.177 | 3.860 |
| 2-Chlorophenol | 0.882 | 0.668 | 0.538 | 0.342 | 4.118 |
| 4-Chlorophenol | 1.016 | 0.794 | 0.886 | 0.205 | 4.802 |
| 3,5-Dimethylphenol | 0.768 | 0.764 | 0.669 | 0.347 | 4.792 |
| T (°C) | PIL00 | PIL10 | PIL20 | PIL30 | PIL40 | PIL60 |
|---|---|---|---|---|---|---|
| 100 | 0.31 | 0.94 | 1.34 | 1.40 | 1.87 | 2.16 |
| 150 | 0.26 | 0.91 | 1.32 | 1.37 | 1.79 | 2.10 |
| 200 | 0.24 | 0.86 | 1.27 | 1.31 | 1.70 | 2.01 |
| 230 | 0.29 a | 0.80 | 1.20 | 1.25 | 1.58 | 1.84 |
| 250 | b | 0.88 a | 1.16 | 1.24 | 1.53 | 1.75 |
| 280 | b | 1.24 a | 1.19 | 1.46 | 1.62 | |
| 300 | b | 1.23 a | 1.52 a | 1.59 | ||
| 320 | b | b | 1.83n a | |||
| 340 | b |
| PIL | T | c | e | s | a | b | l | R2 | n | SE | F |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PIL00 | 60 | −4.10 (0.09) | −0.12 (0.12) | 1.69 (0.11) | 1.72 (0.19) | 0.75 (0.14) | 0.45 (0.03) | 0.98 | 23 | 0.06 | 192 |
| 90 | −3.89 (0.08) | 0.00 (0.11) | 1.55 (0.10) | 1.34 (0.12) | 0.61 (0.15) | 0.42 (0.04) | 0.97 | 21 | 0.06 | 109 | |
| 120 | −4.26 (0.12) | −0.20 (0.17) | 1.31 (0.09) | 1.23 (0.11) | 0.46 (0.08) | 0.40 (0.02) | 0.99 | 21 | 0.04 | 299 | |
| PIL10 | 60 | −4.43 (0.10) | −0.18 (0.09) | 1.81 (0.14) | 1.87 (0.10) | 0.70 (0.08) | 0.62 (0.02) | 0.99 | 23 | 0.02 | 736 |
| 90 | −4.52 (0.10) | −0.40 (0.12) | 1.77 (0.16) | 1.59 (0.17) | 0.55 (0.12) | 0.56 (0.03) | 0.99 | 23 | 0.04 | 242 | |
| 120 | −4.66 (0.09) | −0.41 (0.10) | 1.53 (0.12) | 1.52 (0.09) | 0.39 (0.18) | 0.45 (0.02) | 0.99 | 20 | 0.04 | 221 | |
| PIL20 | 60 | −3.81 (0.13) | 0.06 (0.09) | 1.43 (0.14) | 1.61 (0.14) | 0.66 (0.09) | 0.67 (0.05) | 0.99 | 22 | 0.03 | 393 |
| 90 | −3.85 (0.10) | 0.01 (0.07) | 1.40 (0.10) | 1.24 (0.13) | 0.52 (0.10) | 0.51 (0.04) | 0.98 | 28 | 0.02 | 677 | |
| 120 | −3.90 (0.11) | −0.11 (0.06) | 1.35 (0.09) | 1.15 (0.13) | 0.30 (0.09) | 0.43 (0.03) | 0.99 | 30 | 0.02 | 959 | |
| PIL30 | 60 | −4.21 (0.11) | −0.12 (0.09) | 1.88 (0.12) | 1.84 (0.10) | 0.85 (0.12) | 0.60 (0.03 | 0.99 | 23 | 0.02 | 624 |
| 90 | −4.42 (0.13) | −0.15 (0.16) | 1.53 (0.18) | 1.59 (0.11) | 0.76 (0.14) | 0.56 (0.04) | 0.98 | 22 | 0.04 | 129 | |
| 120 | −4.24 (0.12) | −0.37 (0.11) | 1.41 (0.14) | 1.32 (0.12) | 0.28 (0.13) | 0.45 (0.06) | 0.99 | 21 | 0.03 | 347 | |
| PIL40 | 60 | −4.38 (0.16) | −0.11 (0.12) | 1.62 (0.09) | 1.83 (0.11) | 0.90 (0.08) | 0.54 (0.07) | 0.98 | 27 | 0.02 | 395 |
| 90 | −4.00 (0.15) | −0.13 (0.12) | 1.34 (0.11) | 1.42 (0.10) | 0.27 (0.09) | 0.50 (0.05) | 0.98 | 23 | 0.05 | 131 | |
| 120 | −3.40 (0.19) | −0.40 (0.14) | 0.49 (0.12) | 0.91 (0.09) | 0.11 (0.16) | 0.47 (0.06) | 0.98 | 21 | 0.07 | 112 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, J.; Valls, A.; Arias Abrodo, P.; Gutiérrez Álvarez, M.D.; González-Álvarez, J.; Altava, B.; Luis, S.V. Polymeric Ionic Liquids Derived from L-Valine for the Preparation of Highly Selective Silica-Supported Stationary Phases in Gas Chromatography. Polymers 2020, 12, 2348. https://doi.org/10.3390/polym12102348
González-Rodríguez J, Valls A, Arias Abrodo P, Gutiérrez Álvarez MD, González-Álvarez J, Altava B, Luis SV. Polymeric Ionic Liquids Derived from L-Valine for the Preparation of Highly Selective Silica-Supported Stationary Phases in Gas Chromatography. Polymers. 2020; 12(10):2348. https://doi.org/10.3390/polym12102348
Chicago/Turabian StyleGonzález-Rodríguez, Jorge, Adriana Valls, Pilar Arias Abrodo, María Dolores Gutiérrez Álvarez, Jaime González-Álvarez, Belén Altava, and Santiago V. Luis. 2020. "Polymeric Ionic Liquids Derived from L-Valine for the Preparation of Highly Selective Silica-Supported Stationary Phases in Gas Chromatography" Polymers 12, no. 10: 2348. https://doi.org/10.3390/polym12102348
APA StyleGonzález-Rodríguez, J., Valls, A., Arias Abrodo, P., Gutiérrez Álvarez, M. D., González-Álvarez, J., Altava, B., & Luis, S. V. (2020). Polymeric Ionic Liquids Derived from L-Valine for the Preparation of Highly Selective Silica-Supported Stationary Phases in Gas Chromatography. Polymers, 12(10), 2348. https://doi.org/10.3390/polym12102348