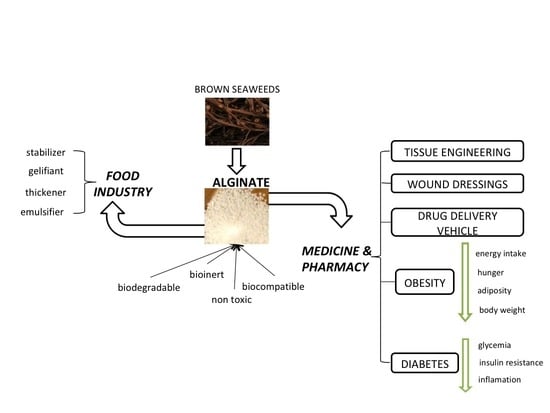
Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders
Abstract
:1. Introduction
2. Alginates: Properties and Challenges
3. Sodium Alginate in the Food Industry
4. Applications of Alginate in the Medical Field
4.1. In Vivo Applications
4.2. Processing Techniques and Applications of Alginate in the Medical Field
4.2.1. Microbeads/Microspheres
Extrusion
Spray-Drying
Emulsification
4.2.2. Wound Dressings
4.2.3. Foam Dressings
4.2.4. Hydrogel Dressings
4.2.5. Alginate Bioaerogels
4.3. Alginate and Tissue Engineering
4.4. Alginate as Drug Delivery Vehicle
4.5. Alginate in Probiotic Encapsulation
4.6. Alginate in the Management of Diabetes
4.7. Alginate in the Management of Obesity
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gundewadi, G.; Rudra, S.; Sarkar, D.; Singh, D. Nanoemulsion based alginate organic coating for shelf life extension of okra. Food Packag. Shelf Life 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Acevendo-Fani, A.; Soliva-Fortuny, R.; Martin-Belloso, O. Nanoemulsions as edible coatings. Curr. Opin. Food Sci. 2017, 15, 43–49. [Google Scholar] [CrossRef]
- Hashemi, S.; Khaneghah, A.; Ghahfarrokhi, M.; Eş, I. Basil-seed gum containing Origanum vulgare subsp. viride essential oilas edible coating for fresh cut apricots. Postharvest Biol. Technol. 2017, 125, 26–34. [Google Scholar] [CrossRef]
- Arroyo, B.; Bezerra, A.; Oliveira, L.; Arroyo, S.; De Melo, E.; Santos, A. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Da Silva, C.; Andrade, L.; Oliveira, D.; Campos, J.; Souto, E. Alginate nanoparticles for drug delivery and targeting. Curr. Pharm. Des. 2019, 25, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, G.; Zhou, Y.; Liu, Y.; Feng, Y.; Li, J. Effects of sodium alginate on microstructural and properties of bacterial cellulose nanocrystal stabilized emulsions. Colloids Surf. A 2020, 607, 125474. [Google Scholar] [CrossRef]
- Martau, G.A.; Mihai, M.; Vodnar, D. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilal, M.; Iqbal, H. Naturally-derived biopolymers: Potential platforms forenzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, M.; Yousefi, H.; Cavallaro, G.; Lazzara, G.; Goh, C.; Lee, S.; Solouk, A.; Pasbakhsh, P. Safely dissolvable and healable active packaging films based on alginate and pectin. Polymers 2019, 11, 1594. [Google Scholar] [CrossRef] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Mantha, S.; Pillai, S.; Khayambasi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.; Tran, S. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.Y.; Park, J.-U.; Choi, M.-H.; Kim, S.; Kim, H.-E.; Jeong, S.-H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 16811. [Google Scholar] [CrossRef]
- Getachew, A.; Jacobsen, C.; Holdt, S. Emerging Technologies for the Extraction of Marine Phenolics: Opportunities and Challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovativetechnologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, A.; Jafari, S.; Tong, Q.; Riaz, T.; Assadpour, E.; Aadil, R.; Niazi, S.; Khan, I.; Shehzad, Q.; Ali, A.; et al. Drug nanodelivery systems based on natural polysaccharides against different diseases. Adv. Colloid Interface Sci. 2020, 284, 102251. [Google Scholar] [CrossRef]
- Van Belleghem, S.; Torres, L.; Santoro, M.; Mahaik, B.; Wolfand, A.; Kofinas, P.; Fisger, P. Hybrid 3D Printing of Synthetic and Cell-Laden Bioinks for Shape Retaining Soft Tissue Grafts. Adv. Funct. Mater. 2020, 30, 1907145. [Google Scholar] [CrossRef] [PubMed]
- Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.; Ismail, A.; Krishna, S.; Berto, F. Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials 2020, 13, 3980. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2019, 115, 459–465. [Google Scholar] [CrossRef]
- Sharma, S.; Sanpui, P.; Chattopadhyay, A.; Sankar Ghosh, S. Fabrication of antibacterial silver nanoparticle—sodium alginate–chitosan composite films. RSC Adv. 2012, 2, 5837–5843. [Google Scholar] [CrossRef]
- Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.; Edgar, K. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Draget, K. Alginates. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Oxford Cambridge: New Delhi, India, 2019; pp. 379–395. [Google Scholar]
- Wang, L.; Yang, S.; Cao, J.; Zhao, S.; Wang, W. Microencapsulation of Ginger Volatile Oil Based on Gelatin/Sodium Alginate Polyelectrolyte Complex. Chem. Pharm. Bull. 2016, 64, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Re-Evaluation of Alginic Acid and Its Sodium, Potassium, Ammonium and Calcium Salts (E 400–E 404) as Food Additives. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5049 (accessed on 2 August 2020).
- Yang, D.; Jones, K. Effect of alginate on innate immune activation of macrophages. J. Biomed. Mater. Res. A 2009, 90, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Xu, X.; Tamura, T.; Oda, T.; Muramatsu, T. Enzymatically depolymerized alginate oligomers that cause cytotoxic cytokine production in human mononuclear cells. Biosci. Biotechnol. Biochem. 2003, 67, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure–activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, N.; Ali Khan, M. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Dinu, V.; Yakubov, G.; Limb, M.; Hurst, K.; Adams, G.; Harding, T.; Fisk, I. Mucin immobilization in calcium-alginate: A possible mucus mimetic tool for evaluating mucoadhesion and retention of flavor. Int. J. Biol. Macromol. 2019, 138, 831–836. [Google Scholar] [CrossRef]
- Sun, J.; Liu, J.; Liu, Y.; Li, Z.; Nan, J. Optimization of Entrapping Conditions of Nitrifying Bacteria and Selection of Entrapping Agent. Procedia Environ. Sci. 2009, 8, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Bourtoom, T. Edible films and coatings: Characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Hammam, A. Technological, applications, and characteristics of edible films and coatings. SN Appl. Sci. 2009, 1, 632. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.; Nilsen-Nygaard, J.; Pettersen, M.; Freire, C. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Natrajan, D.; Srinivasan, S.; Sundar, K.; Ravindran, A. Formulation of essential oil-loaded chitosan-alginate nanocapsules. J. Food Drug Anal. 2015, 23, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Kaur, K.; Kumar, P. Optimizing microencapsulation of a-tocopherol with pectin and sodium alginate. J. Food Sci. Technol. 2018, 55, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Narsaiah, K.; Jha, S.; Wilson, R.; Mandge, H.; Manikantan, N. Optimizing microencapsulation of nisin with sodium alginate and guar gum. J. Food Sci. Technol. 2014, 51, 4054–4059. [Google Scholar] [CrossRef]
- Bierhalz, A.; Da Silva, M.; De Sousab, H.; Braga, M.; Kieckbuscha, T. Influence of natamycin loading methods on the physical characteristics of alginate active films. J. Supercrit. Fluids 2013, 76, 74–82. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, H.; Salama, H.; El-Shafei, K.; Hegazi, M. Micro-encapsulation of Eugenia supra-auxillaris phenolics Rich Fraction for Its Possible Use as a Natural Food Preservative. Egypt J. Chem. 2018, 61, 85–91. [Google Scholar]
- Abd Rahima, S.; Sulaimana, A.; Hamzahb, F.; Ku Hamidb, K.; Muhd Rodhib, K.; Musab, M.; Edamaa, N. Enzymes encapsulation within calcium alginate-clay beads: Characterization and application for cassava slurry saccharification. Procedia Eng. 2013, 68, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Purwantia, N.; Zehna, A.; Pusfitasaric, E.; Khalid, N.; Febriantof, E.; Mardjana, S.; Kobayashi, I. Emulsion stability of clove oil in chitosan and sodium alginate matrix. Int. J. Food Prop. 2018, 21, 566–581. [Google Scholar] [CrossRef] [Green Version]
- Kokina, M.; Shamtsyan, N.; Georgescu, C.; Mironescu, M.; Nedovic, V. Essential oil/alginate microcapsules; obtaining and applying. Immunopathol. Persa 2018, 5, e04. [Google Scholar] [CrossRef]
- Bustos, R.; Alberti, F.; Matiacevich, S. Edible antimicrobial films based on microencapsulated lemongrass oil. J. Food Sci. Technol. 2016, 53, 832–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levic, S.; Raca, V.; Manojlovc, V.; Rakic, V.; Bugarskib, B.; Flockc, T.; Krzyczmonikd, K.; Nedovi, V. Limonene encapsulation in alginate/poly (vinyl alcohol). Procedia Food Sci. 2011, 1, 1816–1820. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium Alginate-Based Green Packaging Films Functionalized by Guava Leaf Extracts and Their Bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.; Miranda, H.; Ribeiro, H.; Rosa, M.; Nascimento, D.M. Nanoreinforced alginate–acerola puree coatings on acerola fruits. J. Food Eng. 2012, 113, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Mania, S.; Tylingo, R.; Michałowska, A. The Drop-in-Drop Encapsulation in Chitosan and Sodium Alginate as a Method of Prolonging the Quality of Linseed Oil. Polymers 2018, 10, 1355. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, N.; Alonso, M.; Saint-Jean, S.; Perez-Prieto, S. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dosedependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish. Shellfish Immunol. 2015, 45, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Liu, C.; Huang, Z.; Xue, F. Preparation and Characterization of Nanoparticles Based on Hydrophobic Alginate Derivative as Carriers for Sustained Release of Vitamin D3. J. Agric. Food Chem. 2011, 59, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, Y.; Xu, Y.; Niu, F.; Li, Z.; Ba, C.; Jin, B.; Chen, G.; Li, X. One-step assembly of zein/caseinate/alginate nanoparticles for encapsulation and improved bioaccessibility of propolis. Food Funct. 2019, 10, 635–645. [Google Scholar] [CrossRef]
- Guo, R.; Du, X.; Zhang, R.; Deng, L.; Dong, A.; Zhang, J. Bio adhesive film formed from a novel organic inorganic hybrid gel for transdermal drug delivery system. Eur. J. Pharm. Biopharm. 2011, 79, 574–583. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Mohamed, N. Alginate-coated caseinate nanoparticles for doxorubicin delivery: Preparation, characterisation, and in vivo assessment. Int. J. Biol. Macromol. 2020, 154, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In vivo anti-inflammatory and anti-allergic activities of cynaroside evaluated by using hydrogel formulations. Biomed. Pharmacother. 2020, 121, 109681. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.; Thach, L.; Tran, M.; Mai, H.; Nguyen, T.; Le, G.; Can, M.; Tran, L.; Bach, G.; et al. Characterization of chitosan/ alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Jia, J.; Wanga, H.; Chen, S.; Cao, W.; Yan, J.; Gong, X.; Lian, X.; Li, W.; Fan, Y. In vitro and in vivo release of diclofenac sodium-loaded sodium alginate/carboxymethyl chitosan-ZnO hydrogel beads. Int. J. Biol. Macromol. 2019, 141, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, S.; Esameili, A. New method of creating hybrid of buprenorphine loaded rifampin/polyethylene glycol/alginate nanoparticles. Int. J. Biol. Macromol. 2020, 159, 204–212. [Google Scholar] [CrossRef]
- Rajpooot, K.; Jain, S. Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: A dual-targeted approach. Int. J. Biol. Macromol. 2020, 151, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; D’Sa, S.; D’Souza, M. Biofabrication of microcapsules encapsulating beta-TC-6 cells via scalable device and in-vivo evaluation in type 1 diabetic mice. Int. J. Biol. Macromol. 2019, 572, 118830. [Google Scholar] [CrossRef]
- Ghumman, S.; Bashir, S.; Noreen, S.; Khan, A.; Malik, M. Taro-corms mucilage-alginate microspheres for the sustained release of pregabalin: In vitro & in vivo evaluation. Int. J. Biol. Macromol. 2019, 139, 1191–1202. [Google Scholar]
- Wu, Y.; Zhang, W.; Huanga, J.; Luoa, Z.; Li, J.; Wanga, L.; Di, L. Mucoadhesive improvement of alginate microspheres as potential gastroretentive delivery carrier by blending with Bletilla striata polysaccharide. Int. J. Biol. Macromol. 2020, 156, 1191–1201. [Google Scholar] [CrossRef]
- Kong, F.; Fan, C.; Yanga, Y.; Lee, B.; Wei, K. 5-hydroxymethylfurfural-embedded poly (vinyl alcohol)/sodium alginate hybrid hydrogels accelerate wound healing. Int. J. Biol. Macromol. 2019, 138, 933–949. [Google Scholar] [CrossRef]
- Wang, Y.; Fanb, S.; Li, B.; Niu, G.; Li, X.; Guoa, Y.; Zhang, J.; Shi, J.; Wang, X. Silk fibroin/sodium alginate composite porous materials with controllable degradation. Int. J. Biol. Macromol. 2020, 150, 1314–1322. [Google Scholar] [CrossRef]
- Nazarnezhada, S.; Abbaszadeh-Goudarzi, G.; Samadian, H.; Khaksari, M.; Ghatar, J.; Khastar, H.; Rezaei, D.; Mousavi, S.; Shirian, S.; Salehi, M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 164, 3323–3331. [Google Scholar] [CrossRef]
- García-García, P.; Rezes, R.; Pérez-Herrero, E.; Arnau, M.; Evora, C.; Delgado, A. Alginate-hydrogel versus alginate-solid system. Efficacy in bone regeneration in osteoporosis. Mater. Sci. Eng. C 2020, 115, 111009. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Q.; Xie, S.; Shan, X.; Cai, Z. In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatinsodium alginate/rat Schwann-cell scaffold. Mater. Sci. Eng. C 2020, 109, 110530. [Google Scholar] [CrossRef] [PubMed]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C 2020, 113, 110957. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. Part A 2020, 108, 545–556. [Google Scholar] [CrossRef]
- Salehi, M.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Ebrahimi-Barough, S. Accelerating healing of excisional wound with alginate hydrogel containing naringenin in rat model. Drug Deliv. Transl. Res. 2020. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGFencapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Feng, T.; Li, B.; Han, Y. In Vitro and in Vivo Comparison Study of Electrospun PLA and PLA/PVA/SA Fiber Membranes for Wound Healing. Polymers 2020, 12, 839. [Google Scholar] [CrossRef] [Green Version]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.; et al. Growth Factors Delivery System for Skin Regeneration: An Advanced Wound Dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Diniz, F.; Maia, R.; Andrade, L.; Andrade, L.N.; Chaud, M.; Da Silva, C.; Corrêa, C.; De Albuquerque Junior, R.; Da Costa, L.P.; Shin, S.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing in Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lueckgen, A.; Garske, D.; Ellinghaus, A.; Mooney, D.; Duda, G.; Cipitria, A. Enzymatically-degradable alginate hydrogels promote cell spreading and in vivo tissue infiltration. Biomaterials 2019, 217, 119294. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Dawei, C.; Liping, X.; Rongqing, Z. Oral colon-specific drug delivery for bee venom peptide: Development of a coated calcium alginate gel beads-entrapped liposome. J. Control. Release 2003, 93, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Martins, J.; Hirvonen, J.; Santos, H. Spray-drying for the formulation of oral drug delivery systems. In Nanotechnology for Oral Drug Delivery, 1st ed.; Martins, J., Santos, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 253–283. [Google Scholar]
- Thanh Uyen, N.; Abdul Hamid, Z.; Thanh Tram, N.; Ahmad, N. Fabrication of alginate microspheres for drug delivery: A review. Int. J. Biol. Macromol. 2020, 153, 1035–1046. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.; Assadpour, E. Development of a nutraceutical nano-delivery system through emulsification/internal gelation of alginate. Food Chem. 2017, 229, 286–295. [Google Scholar] [CrossRef]
- Gutierrez, E.; Burdiles, P.; Quero, F.; Palma, P.; Olate-Moya, F.; Palza, H. 3D Printing of antimicrobial alginate/bacterial-cellulose composite hydrogels by incorporating copper nanostructures. ACS Biomater. Sci. Eng. 2019, 5, 6290–6299. [Google Scholar] [CrossRef]
- Qin, Y. The gel swelling properties of alginate fibers and their applications in wound management. Adv. Polym. Technol. 2018, 19, 6–14. [Google Scholar] [CrossRef]
- Deepthi, S.; Jayakumar, R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact. Mater. 2018, 3, 194–200. [Google Scholar] [CrossRef]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.B.; Dhakate, S.R. In vivo wound healing performance of drug loaded electrospin composite nanofibers transdermal patch. Int. J Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef]
- Bindu, T.; Vidyavathi, M.; Kavitha, K.; Sastry, T.; Suresh Kumar, R. Preparation and evaluation of chitosan-gelatin composite films for wound healing activity. Trends Biomater. Artif. Organs 2010, 24, 123–130. [Google Scholar]
- Henry, B.; Neill, D.; Becker, K.; Gore, S.; Bricio-Moreno, L.; Ziobro, R.; Edwards, M.; Mühlemann, K.; Steinmann, J.; Kleuser, B.; et al. Engineered liposomes sequester bacterial exotoxins and protect from severe invasive infections in mice. Nat. Biotechnol. 2014, 33, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okur, N.Ü.; Hökenek, N.; Okur, M.; Ayla, S.; Yoltas, A.; Siafaka, P.; Cevher, E. An alternative approach to wound healing field; new composite films from natural polymers for mupirocin dermal delivery. Saudi Pharm. J. 2019, 27, 738–752. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.A.; Lai, C.S.; Corwin, H.; Ma, Y.; Maki, K.C.; Garleb, K.A.; Wolf, K.A. Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J. Nutr. 2004, 134, 886–889. [Google Scholar] [CrossRef] [Green Version]
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2014, 32, 462–467. [Google Scholar]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, D.W.; Kim, D.S.; Kim, J.O.; Yong, C.S.; Cho, K.H.; Youn, Y.S.; Jin, S.G.; Choi, H.G. Novel neomycin sulfate-loaded hydrogel dressing with enhanced physical dressing properties and wound-curing effect. Drug Deliv. 2016, 23, 2806–2812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Melvik, J.; Gåserød, O.; Alsberg, E.; Christensen, B. Ionically Gelled Alginate Foams: Physical Properties Controlled by Operational and Macromolecular Parameters. Biomacromolecules 2012, 13, 3703–3710. [Google Scholar] [CrossRef]
- Mohamad, N.; Mohd Amin, M.C.I.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Yasasvini, S.; Anusa, R.S.; VedhaHari, B.N.; Prabhu, P.C.; RamyaDevi, D. Topical hydrogel matrix loaded with Simvastatin microparticles for enhanced wound healing activity. Mater. Sci. Eng. C 2017, 72, 160–167. [Google Scholar] [CrossRef]
- Raval, J.; Naik, D.; Patel, P. Preparation and evaluation of gatifloxacin dermal patches as wound dressings. Int. J. Drug Formul. Res. 2011, 2, 247–259. [Google Scholar]
- Siafaka, P.I.; Barmbalexis, P.; Bikiaris, D.N. Novel electrospun nanofibrousmatrices prepared from poly (lactic acid)/poly (butylene adipate) blends forcontrolled release formulations of an anti-rheumatoid agent. Eur. J. Pharm. Sci. 2016, 88, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kurczewska, J.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Halloysite nanotubes as carriers of vancomycin in alginate-based wound dressing. Saudi Pharm. J. 2017, 25, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Capeling, M.; Czerwinski, M.; Huang, S.; Tsai, S.-H.; Wu, A.; Nagy, M.; Juliar, B.; Sundaram, N.; Song, Y.; Han, W.; et al. Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep. 2019, 12, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe vera hydrogel films for biomedical applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Alemán, J.; Chadwick, A.; He, J.; Hess, M.; Horie, K.; Jones, R.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2017, 79, 1801–1829. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- El-Nahhal, I.; El-Ashgar, N.M. A review on polysiloxane-immobilized ligand systems: Synthesis, characterization and applications. J. Organomet. Chem. 2007, 692, 2861–2886. [Google Scholar] [CrossRef]
- Mikkonen, K.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Hajipour, M.; Barzegari, E.; Lotfabadi, A.; Ferdousi, M.; Saboury, A.; Raoufi, M.; Awala, H.; Mintova, S. Zeolite nanoparticles inhibit Aβ–fibrinogen interaction and formation of a consequent abnormal structural clot. ACS Appl. Mater. Interfaces 2016, 8, 30768–30779. [Google Scholar] [CrossRef] [PubMed]
- Poorakbar, E.; Shafiee, A.; Saboury, A.; Rad, B.; Khoshnevisan, K.; Ma’mani, L.; Derakhshankhah, H.; Ganjali, M.; Hosseini, M. Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: Improvement of enzymatic activity and thermal stability. Process. Biochem. 2018, 71, 92–100. [Google Scholar] [CrossRef]
- Macan, J. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials. Kem. Ind. 2011, 60, 135–153. [Google Scholar]
- Soorbaghia, F.; Isanejadb, M.; Salatinc, S.; Ghorbanie, M.; Jafarif, S.; Derakhshankhahf, H. Bioaerogels: Synthesis approaches, cellular uptake, and the biomedical applications. Biomed. Pharmacother. 2019, 111, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Ruban, S. Biobased packaging-application in meat industry. Vet. World 2009, 2, 79–82. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Koga, H.; Qi, Z.; Saito, T.; Isogai, A. Nanofibrillar chitin aerogels as renewable base catalysts. Biomacromolecules 2014, 15, 4314–4319. [Google Scholar] [CrossRef]
- Cai, H.; Sharma, S.; Liu, W.; Mu, W.; Liu, W.; Zhang, X.; Deng, Y. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules 2014, 15, 2540–2547. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Sudo, H.; Todoh, M.; Yamada, K.; Iwasaki, K.; Ohnishi, T.; Hirohama, N.; Nonoyama, T.; Ukeba, D.; Ura, K.; et al. An acellular bioresorbable ultra-purified alginate gel promotes intervertebral disc repair: A preclinical proof-of-concept study. EBioMedicine 2018, 37, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Ruvinov, E.; Re’em, T.; Witte, F.; Cohen, S. Articular cartilage regeneration using acellular bioactive affinity-binding alginate hydrogel: A 6-month study in a mini-pig model of osteochondral defects. J. Orthop. Transl. 2019, 16, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, T.; Sah, R.L. Biomechanics of integrative cartilage repair. Osteoarthr. Cartil. 1999, 7, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król, Z.; Malik, M.; Marycz, M.; Jarmoluk, A. Physicochemical Properties of Biopolymer Hydrogels Treated by Direct Electric Current. Polymers 2016, 8, 248. [Google Scholar] [CrossRef]
- Freitas, R., Jr. What is nanomedicine? Nanomedicine 2005, 1, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Ghune, M.; Jain, D. Nano-medicine based drug delivery system. J. Adv. Pharm. Techol. Res. 2011, 1, 201–213, ISSN 2249-3379. [Google Scholar]
- Friedman, A.; Phan, J.; Schairer, D.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.; Modlin, R.; et al. Antimicrobial and Anti-Inflammatory Activity of Chitosan–Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Invest. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, G.; Gupta, P.; Bansal, A. Gastroretentive drug delivery system. In Progress in Controlled and Novel Drug Delivery; Jain, N.K., Ed.; CBS: New Delhi, India, 2004; pp. 76–97. [Google Scholar]
- Jagdale, S.; Agavekar, A.; Pandya, S.; Kuchekar, B.; Chabukswar, A. Formulation and Evaluation of Gastroretentive Drug Delivery System of Propranolol Hydrochloride. AAPS Pharmacol. Sci. Technol. 2009, 10, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.; Washington, N. The stomach: Its role in oral drug delivery. In Physiological Pharmaceutical: Biological Barriers to Drug Absorption; Ellis Horwood: Chichester, UK, 1989; pp. 47–70. [Google Scholar]
- Nayak, A.; Maji, R.; Das, B. Gastroretentive drug delivery systems: A review. Asian J. Pharm. Clin. Res. 2010, 3, 1–9, ISSN 0974-2441. [Google Scholar]
- Ali, M.; Bakalis, S. Mucoadhesive polymers for food formulations. Procedia Food Sci. 2011, 1, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.; Woods, S.; Methven, L.; Parker, J.; Khutoryanskiya, V. Mucoadhesive polysaccharides modulate sodium retention, release and taste perception. Food Chem. 2018, 240, 482–489. [Google Scholar] [CrossRef]
- Govender, M.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Van Vuuren, S.; Pillay, V. A review of the advancements in probiotic delivery: Conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS Pharm. Sci. Tech. 2014, 15, 29–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, G.; Malone, M.; Homan, J.; Norton, I. A mathematical model of volatile release in mouth from the dispersion of gelled emulsion particles. J. Control. Release 2004, 98, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Appelqvist, I. Gelled emulsion particles for the controlled release of lipophilic volatiles during eating. J. Control. Release 2003, 90, 227–241. [Google Scholar] [CrossRef]
- Cheng, B.; Li, D.; Huo, O.; Zhao, Q.; Lan, Q.; Cui, M.; Pan, W.; Yang, X. Two kinds of ketoprofen enteric gel beads (CA and CS-SA) using biopolymer alginate. Asian J. Pharm. Sci. 2018, 13, 120–130. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.-S. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2015, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Sarei, F.; Dounighi, N.M.; Zolfagharian, H.; Khaki, P.; Bidhendi, S.M. Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Indian J. Pharm. Sci. 2013, 75, 442–449. [Google Scholar] [PubMed] [Green Version]
- Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 10 August 2020).
- Cook, M.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niness, K.R. Inulin and oligofructose: What are they? J. Nutr. 1999, 129, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Douglas, L.C.; Sanders, M.E. Probiotics and prebiotics in dietetics practice. J. Am. Diet. Assoc. 2008, 108, 510–521. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Fu, N.; Chen, X.D. Towards a maximal cell survival in convective thermal drying processes. Food Res. Int. 2011, 44, 1127–1149. [Google Scholar] [CrossRef]
- D’Orazio, G.; Gennaro, P.; Boccarusso, M.; Presti, I.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Labra, M.; La Ferla, B. Microencapsulation of new probiotic formulations for gastrointestinal delivery: In vitro study to assess viability and biological properties. Appl. Microbiol. Biotechnol. 2015, 99, 9779–9789. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food BioSci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Pan-utai, W.; Iamtham, S. Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. J. King Saud Univ. Sci. 2018, 31, 1535–1542. [Google Scholar] [CrossRef]
- Sunny-Roberts, E.; Knorr, D. The protective effect of monosodium glutamate on survival of Lactobacillus rhamnosus GG and Lactobacillus rhamnosus E-97800 (E800) strains during spray-drying and storage in trehalose containing powders. Int. Dairy J. 2009, 19, 209–214. [Google Scholar] [CrossRef]
- Yonekura, L.; Sun, H.; Soukoulis, C.; Fisk, I. Microencapsulation of Lactobacillus acidophilus NCIMB 701748 in matrices containing soluble fiber by spray drying: Technological characterization, storage stability and survival after in vitro digestion. J. Funct. Foods 2014, 6, 205–214. [Google Scholar] [CrossRef]
- Malmo, C.; La Storia, A.; Mauriello, G. Microencapsulation of Lactobacillus reuteri DSM 17938 Cells Coated in Alginate Beads with Chitosan by Spray Drying to Use as a Probiotic Cell in a Chocolate Soufflé. Food Bioprocess. Technol. 2013, 6, 795–805. [Google Scholar] [CrossRef]
- Liu, H.; Gong, J.; Chabot, D.; Miller, S.; Cui, S.; Zhong, F.; Wang, Q. Improved survival of Lactobacillus zeae LB1 in a spray dried alginate-protein matrix. Food Hydrocoll. 2017, 78, 100–108. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Y.; Xu, N.; Mu, H.; Zhang, H.; Duan, J. Improved viability of Akkermansia muciniphila by encapsulation in spray dried succinate-grafted alginate doped with epigallocatechin-3-gallate. Int. J. Biol. Macromol. 2020, 159, 373–382. [Google Scholar] [CrossRef]
- Etchepare, M.; Raddatz, G.; De Moraes Flores, E.; Zepka, L.; Jacob-Lopes, E.; Barin, J.; Grosso, C.; De Menezes, C. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT Food Sci. Technol. 2016, 65, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Govender, S.; Pillay, V.; Chetty, D.J.; Essack, S.Y.; Dangor, C.M.; Govender, T. Optimisation and Characterisation of Bioadhesive Controlled Release Tetracycline Microspheres. Int. J. Pharm. 2005, 306, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Bueno, L.; De Aguiar Júnior, F.; Finkler, C. Preparation and characterization of alginate and gelatin microcapsules containing Lactobacillus rhamnosus. An. Acad. Bras. Ciências 2017, 89, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Holkem, A.; Raddatz, C.; Barin, J.; Moraes Flores, E.; Muller, E.; Codevilla, C.; Jacob-Lopes, E.; Ferreira Grosso, C.; De Menezes, C. Production of microcapsules containing Bifidobacterium BB-12 by emulsification/internal gelation. LWT Food Sci. Technol. 2017, 76, 216–221. [Google Scholar] [CrossRef]
- Klinkenberg, G.; Lystad, K.; Levine, D.; Dyrset, N. Cell Release from Alginate Immobilized Lactococcus lactis ssp. lactis in Chitosan and Alginate Coated Beads. J. Dairy Sci. 2001, 84, 1118–1127. [Google Scholar] [CrossRef]
- Pereira, J.; Soares, J.; Costa, E.; Silva, S.; Gomes, A.; Pintado, M. Characterization of Edible Films Based on Alginate or Whey Protein Incorporated with Bifidobacterium animalis subsp. lactis BB-12 and Prebiotics. Coatings 2019, 9, 493. [Google Scholar] [CrossRef] [Green Version]
- Astesana, D.; Zimmermanna, J.; Frizzoa, L.; Zbruna, M.; Blajmana, J.; Berisvil, A.; Romero-Scharpena, A.; Signorinib, M.; Rosmini, M.; Sotoa, L. Development and storage studies of high density macrocapsules containing Lactobacillus spp. strains as nutritional supplement in young calves. Rev. Argent. Microbiol. 2018, 50, 398–407. [Google Scholar] [CrossRef]
- Qaziyani, S.; Pourfarzad, A.; Gheibi, S.; Roozbeh Nasiraie, L. Effect of encapsulation and wall material on the probiotic survival and physicochemical properties of synbiotic chewing gum: Study with univariate and multivariate analyses. Helyion 2019, 5, e02144. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.; Retamales, J.; Palza, H.; Bastías, R. Encapsulation of specific Salmonella Enteritidis phage f3αSE on alginate-spheres as a method for protection and dosification. Electron. J. Biotech. 2018, 31, 57–60. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef]
- Ji, R.; Wu, J.; Zhang, J.; Wang, T.; Zhang, T.; Shao, L.; Chen, D.; Wang, J. Extending Viability of Bifidobacterium longum in Chitosan-Coated Alginate Microcapsules Using Emulsification and Internal Gelation Encapsulation Technology. Front. Microbiol. 2019, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Yonekura, L.; Gan, H.H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ma, D.; Qin, W.; Liu, W. Physical and Antibacterial Properties of Sodium Alginate—Sodium Carboxymethylcellulose Films Containing Lactococcus lactis. Molecules 2018, 23, 2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kailasapathy, K.; Chin, J.C. Survival and therapeutic potential of probiotics organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Cardello, A.; Snow, C.; Schutz, H.; Lesher, L. Predictors of food acceptance, consumption and satisfaction in specific eating situations. J. Food Qual. 2000, 11, 201–216. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhousea, D.; Waterhousea, G.; Cuia, C.; Ruana, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 21 August 2020).
- Li, M.; Sun, Y.; Ma, C.; Hua, Y.; Zhang, L.; Shen, J. Design and Investigation of Penetrating Mechanism of Octaarginine-Modified Alginate Nanoparticles for Improving Intestinal Insulin Delivery. J. Pharm. Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Izeia, L.; Eufrasio-da-Silva, T.; Dolatshahi-Pirouz, A.; Ostrovidov, S.; Paolone, G.; Peppas, N.; De Vos, P.; Dwaine, E.; Orive, G. Cell-laden alginate hydrogels for the treatment of diabetes. Expert Opin. Drug Deliv. 2020, 17, 1113–1118. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Mackie, A.; Macierzanka, A.; Aarak, K.; Rigby, N.; Parker, R.; Channell, G.; Harding, S.; Bajka, B. Sodium alginate decreases the permeability of intestinal mucus. Food Hydrocoll. 2016, 52, 749–755. [Google Scholar] [CrossRef]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Wilcox, M.; Chater, P.; Brownlee, I.; Seal, C.; Pearson, J. Biological activity of alginate and its effect on pancreatic lipase inhibition as a potential treatment for obesity. Food Hydrocoll. 2015, 49, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chater, P.; Wilcox, M.; Brownlee, I.; Pearson, J. Alginate as a protease inhibitor in vitro and in a model gut system; selective inhibition of pepsin but not trypsin. Carbohydr. Polym. 2015, 131, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.; Marques, A.; Pastrana, L.; Teixeira, J.; Sillankorva, S.; Cerqueira, M. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, M.; Brownlee, I.; Richardson, C.; Dettmar, P.; Pearson, P. The modulation of pancreatic lipase activity by alginates. Food Chem. 2014, 146, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.; Guarino, M.; Barosso, S.; Gil, M. Phytopharmacological Strategies in the Management of Type 2 Diabetes Mellitus. Foods 2020, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Paxman, J.; Richardson, J.; Dettmar, P.; Corfe, B. Alginate reduces the increased uptake of cholesterol and glucose in overweight male subjects: A pilot study. Nutr. Res. 2008, 28, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Houghton, D.; Wilcox, M.; Brownlee, I.; Chater, P.; Seal, C.; Pearson, J. Acceptability of alginate enriched bread and its effect on fat digestion in humans. Food Hydrocoll. 2019, 93, 395–401. [Google Scholar] [CrossRef]
- Jensen, M.; Kristensen, M.; Belza, A.; Knudsen, J.; Astrup, A. Acute Effect of Alginate-Based Preload on Satiety Feelings, Energy Intake, and Gastric Emptying Rate in Healthy Subjects. Obesity 2012, 20, 1851–1858. [Google Scholar]
- Maljaars, P.; Peters, H.; Mela, D.; Masclee, A. Ileal brake: A sensible food target for appetite control. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef]
- Wolf, B.W.; Lai, C.S.; Kipnes, M.S.; Ataya, D.G.; Wheeler, K.B.; Zinker, B.A. Glycemic and insulinemic responses of nondiabetic healthy adult subjects to an experimental acid-induced viscosity complex incorporated into a glucose beverage. Nutrition 2002, 18, 621–626. [Google Scholar] [CrossRef]
- Rayment, P.; Wright, P.; Hoad, C.; Ciampi, E.; Haydock, D.; Gowland, P.; Butler, M. Investigation of alginate beads for gastro-intestinal functionality, Part 1: In vitro characterisation. Food Hydrocoll. 2009, 23, 816–822. [Google Scholar] [CrossRef]
- Corstens, M.; Berton-Carabin, C.; Elichiry-Ortiz, P.; Hol, K.; Troost, F.; Masclee, A.; Schroën, K. Emulsion-alginate beads designed to control in vitro intestinal lipolysis: Towards appetite control. J. Funct. Foods 2017, 34, 319–328. [Google Scholar] [CrossRef]



| Product | Substances Incorporated | Effects | References |
|---|---|---|---|
| Chitosan-alginate nanocapsules | turmeric EO and lemongrass EO | Nanocapsules were hemocompatible and used in biomedical and pharmaceutical applications; low and sustained release at neutral pH over 48 h. | [34] |
| SA and pectin | a-tocopherol | Antioxidant in bakery products. Prevents auto-oxidation and increases the shelf life. Encapsulation facilitates handling, enhances stability and maintains prolonged release. | [35] |
| SA and guar gum | nisin | Used as material for nisin encapsulation. Bactericidal effect and possibility to be introduced into the food system. | [36] |
| Alginate films | natamycin | May be used as antimicrobial packaging; are homogeneous, visually attractive, translucent and can be easily processed by different incorporation methods. | [37] |
| SA | Eugenia supra-auxillaris microencapsulation | The encapsulation efficiency was 82%; the microcapsules can be used as food preservatives; maintains the antimicrobial activity against B. subtilis, B. cereus, P. aeruginosa, S aureus and A. niger. | [38] |
| Chitosan and SA matrix | clove oil | The emulsion system was stable; separation of the phase occurred after 28 days of storage. | [39] |
| Calcium alginate-clay beads used for the saccharification of cassava slurry into glucose | multienzymes (alpha-amylase, glucoamylase and cellulase | Under optimal conditions, the immobilization yields and the loading efficiency of enzymes were 97.07%. The beads maintained 51.77% of the residual enzyme’s activity after seven hydrolysis cycles. | [40] |
| Alginate microcapsules | seasoning EO | Due to bioactive and flavoring properties, the EO microcapsules can be incorporated into functional foods. The safety and the sensorial properties of foods through the addition of natural flavorings and preservatives can be improved. | [41] |
| Edible alginate film | lemongrass oil | Films with lemongrass oil concentrations of 1250, 2500 and 5000 ppm inhibit growth of L. monocytogenes and E. coli. Practical application of these films for shelf life extension of fish, meat or cheese. | [42] |
| Alginate/PVOH capsules | limenone | Stability of encapsulated d-limonene in comparison with free aroma; the mixture alginate-polyvinyl alcohol represents an efficient aroma encapsulation matrix. | [43] |
| Chitosan and alginate microcapsules | cinnamon EO | Microcapsules were of uniform size, with a sustained release of EO exceeding 168 h. | [44] |
| SA-Based Green Packaging Films | guava leaf extracts | Enhanced antioxidant and antibacterial abilities of packaging material; the results encourage the use of agricultural byproducts that provide functional ingredients. | [45] |
| Alginate coating | acerola puree | The alginate-acerola puree coating extended fruit stability by decreasing ascorbic acid and weight loss, decay incidence and by delaying the ripening process. | [46] |
| Chitosan and SA capsule | linseed oil | Quality of oil increased after encapsulation; chitosan and sodium alginate hydrogel can be used to protect food ingredients stored in aquatic environments such as linseed oil. | [47] |
| Alginate microspheres | oral DNA vaccine against IHNV | The vaccine reduced the virus incidence in the tissues of vaccinated fish. After the oral administration of increasing concentrations of a DNA vaccine against IHNV, there was a significant increase in fish immune responses and resistance to an IHNV infection. | [48] |
| Alginate | vitamin D3 | Liposoluble nutraceuticals are incorporated in alginate nanocapsules, with sustained release in gastrointestinal fluid. | [49] |
| Zein/caseinate/ alginate nanocapsules | propolis | The bioaccessibility of propolis encapsulated in nanocapsules was improved by 80% compared to free propolis (aprox. 30%). | [50] |
| Biopolymer | In Vivo Applications | Results | References |
|---|---|---|---|
| Caseinate nanoparticles loaded with DOX coated with alginate | tumor-bearing mice | Nanoparticles facilitated controlled and sustained drug releasing and enhanced DOX effectiveness against Ehrlich carcinoma. | [53] |
| Alginate hydrogel with cianoside | mouse skin with inflammation or atopic dermatitis | Alginate reduced the number of T cells, mast cells and histiocytes, paw skin, ear tissue inflammation, and inflammatory infiltrates. | [54] |
| Chitosan/alginate/lovastatin nanoparticles | adult healthy Swiss mice | Formulated as a new drug carrier, the nanoparticles were safe, nontoxic and could be applied to lower serum cholesterol. | [55] |
| SA-based hydrogel beads with diclofenac sodium | Wistar rats | Good delivery system for drugs that could irritate the stomach, such as diclofenac sodium. | [56] |
| Buprenorphine-loaded rifampin/polyethylene glycol/alginate nanoparticles | Wistar rats | Decreased drug dose consumption and liver tissue damage. | [57] |
| Folic acid-grafted solid lipid nanoparticles incorporated in alginate microbeads | Balb/c mice | Coated microbeads released IHT in the colon region next to tumors, with efficiency in treatment of colorectal cancer. Showed antitumor effects against HT-29 cells. | [58] |
| Alginate microcapsules with Beta-TC-6 cells | diabetic mice | Although the microcapsules restored normoglycemia in diabetic mice, the effects were lost after 35 days. | [59] |
| Pregabalin alginate-taro corms mucilage microspheres | male albino rabbits | Blended microspheres increased bioavailability and half-life, being an emerging potential pharmaceutical excipient for sustained drug release. | [60] |
| Bletilla striata—SA microspheres | male Sprague-Dawley rats | Good gastroretentive drug delivery system due to strong adhesion to gastric mucosa and long resistance time in the stomach. | [61] |
| 5-HMF and silver nanoparticles incorporated in PVOH/SA hydrogels | male Sprague-Dawley rats | Hydrogel accelerated wound healing, neovascularization, wound closure, promoting re-epithelization and collagen deposition. | [62] |
| Silk fibroin/SA composite porous materials | male Sprague-Dawley rats | Subcutaneous implantation materials were infiltrated, and, although well tolerated, they largely lost their structural integrity after 21 days. | [63] |
| Alginate hydrogel with H2S as wound dressing material | Wistar rats | Treatment facilitated formation of sebaceous glands, hair follicles and complete epithelialization, without fibroplasia or inflammation. | [64] |
| β-estradiol and BMP-2 alginate scaffolds | osteoporotic and nonosteoportic rats | Without effect in bone mineralization and bone regeneration process. | [65] |
| 3D bioprinted gelatin SA scaffold | Rat Schwann cells | The construct maintained viability and promoted adhesion of Schwann cells, with good biocompatibility and improved cell adhesion. | [66] |
| Freeze-gelled alginate/gelatin scaffolds | Wistar rats | Scaffolds contributed to the wound healing process, by collagen synthesis and remodeling, with rejuvenation of hair follicles and skin appendages. | [67] |
| Exosome—alginate based hydrogel | Wistar rats | The composite enhanced wound closure, re-epithelization, collagen deposition, and angiogenesis at the wound beds. | [68] |
| Naringenin - alginate hydrogel | Wistar rats | The wounds were almost healed after two weeks. | [69] |
| PVOH/ SA hydrogel-based scaffold with bFGF-encapsulated microspheres incorporated | Wistar rats | Healing process was accelerated due to epithelialization, collagen deposition and antimicrobial effect, by inhibiting S. aureus and E. coli growth. | [70] |
| PLA/PVOH/SA | Sprague-Dawley male rats | Positive effects on collagen deposition, angiogenesis and inflammation, and reduced the inflammatory responses during early wound healing. | [71] |
| Alginate and growth factors | C57/BL6 mice | The composite promoted the healing process, formation of granulation tissue, new collagen deposition and rapid skin regeneration. | [72] |
| Alginate/gelatine/silver nanoparticles | adult females Wistar rats | Nanoparticles accelerated tissue formation and promoted earlier development of primary collagen scars. | [73] |
| Norbornene-modified alginate | female C57/Bl6 mice | Due to its good tissue and cell infiltration process, it can be useful in tissue engineering, such as regeneration and drug delivery. | [74] |
| Product | Microencapsulated Strains | Characteristics | References |
|---|---|---|---|
| Alginate and gelatin microcapsules | L. rhamnosus | The concentration of viable cells decreased with an increase in the concentration of the polymers; cell resistance of L. rhamnosus (105 CFU g/L) exceeded four months. | [149] |
| SA microcapsules | Bifidobacterium BB-12 | After 120 days of cold storage (−18 °C), 7.31 log CFU g−1; stability increased with decrease in temperature. | [150] |
| Chitosan and alginate beads | L. lactis ssp. lactis | Subjected to milk fermentation, coating had a significant effect on the rate of cell release within 50 h of continuous fermentation. | [151] |
| Edible films based on alginate or whey protein | B. animalis subsp. lactis BB-12 and prebiotics (inulin and fructooligosaccharides) | Viability was maintained within the minimum threshold (106 CFU/g) necessary to act as a probiotic during 60 days of storage at 23 °C. Incorporation of prebiotic compounds improved B. animalis subsp. lactis BB-12 viability, with inulin showing the best performance; viability was maintained at 7.34 log CFU/g. | [152] |
| Calcium alginate macrocapsules | L. casei DSPV318T and L. plantarum DSPV354T | Refrigeration maintained concentration above 109 CFU/capsule until day 70, and storage at −20 °C showed counts above 109 CFU/capsule until the end of the study (84 days). | [153] |
| Symbiotic chewing gum | L. reuteri | After 21 days, the number of L. reuteri in the encapsulated probiotic chewing gum was higher than in the free probiotic. | [154] |
| Starch, chitosan and alginate microencapsulation | L.acidophilus | Lyophilized microparticles showed values above 6 log CFU g−1 at cold and frozen temperatures, counts within the range for probiotics for 60 days of storage. | [145] |
| Alginate-spheres | S. enteritidis phage f3αSE | Encapsulation in alginate-Ca+2 spheres extended viability. Used as a phage dosification method in water flow systems (phage concentration 102–104 PFU/mL during 250 h). | [155] |
| SA, pectin, carrageenan and gelatin edible films | L. rhamnosus | Storage stability (over 25 days) of L. rhamnosus at both tested temperatures (4 and 25 °C), in descending order, was carrageenan > sodium alginate > gelatin > pectin. | [156] |
| SA, chitosan and HPMC | L. acidophilus NCIMB 701748 | Inactivation rates of L. acidophilus NCIMB 701748 in powders stored at 25 °C were in the following order: HPMC > control > alginate >>> chitosan. | [143] |
| Chitosan-coated alginate microcapsules | B. longum | Chitosan-coated alginate microcapsules protected B. longum from gastrointestinal fluid and high-temperature conditions. | [157] |
| Probiotic baked cereal (with SA) | L. rhamnosus GG | Use of air-dried probiotic sodium alginate film improved viability of L. rhamnosus GG under simulated gastrointestinal conditions. A bread slice delivered ~7.57–8.98 and 6.55–6.91 log CFU/portion before and after in-vitro digestion. | [158] |
| SA—sodium CMC films | L. lactis | Films showed significant bacteriostatic activity against S. aureus at refrigeration conditions for up to one week. | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghita Puscaselu, R.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers 2020, 12, 2417. https://doi.org/10.3390/polym12102417
Gheorghita Puscaselu R, Lobiuc A, Dimian M, Covasa M. Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers. 2020; 12(10):2417. https://doi.org/10.3390/polym12102417
Chicago/Turabian StyleGheorghita Puscaselu, Roxana, Andrei Lobiuc, Mihai Dimian, and Mihai Covasa. 2020. "Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders" Polymers 12, no. 10: 2417. https://doi.org/10.3390/polym12102417
APA StyleGheorghita Puscaselu, R., Lobiuc, A., Dimian, M., & Covasa, M. (2020). Alginate: From Food Industry to Biomedical Applications and Management of Metabolic Disorders. Polymers, 12(10), 2417. https://doi.org/10.3390/polym12102417


.png)