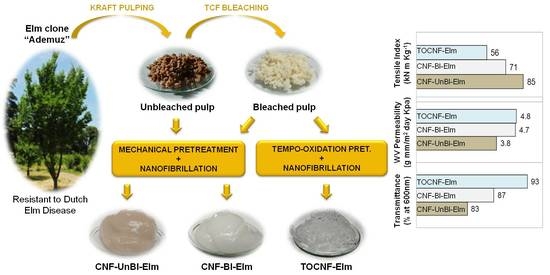
Cellulose Nanofibers from a Dutch Elm Disease-Resistant Ulmus minor Clone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Production of Unbleached and Bleached Elm Pulps
2.3. Production of Cellulose Nanofibers (CNFs) by Mechanical Methods
2.3.1. Mechanical Pretreatment: Refining in PFI Mill
2.3.2. Microfluidization
2.4. Production of TEMPO-Mediated Oxidized Cellulose Nanofibers (TOCNFs)
2.4.1. Chemical Pretreatment: TEMPO-Mediated Oxidation
2.4.2. Microfluidization
2.5. Nanopaper Preparation
2.6. Characterization of Pulps, Cellulose Nanofibers and Nanopapers
2.6.1. Chemical Composition
2.6.2. Carboxylate Content
2.6.3. Fibrillation Yield
2.6.4. Zeta-Potential Measurement
2.6.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6.6. X-Ray Powder Diffraction (XRD) Analysis
2.6.7. Thermogravimetric Analysis (TGA)
2.6.8. Mechanical Properties
2.6.9. Water Vapor Sorption Isotherms
2.6.10. Water Vapor Permeability
2.6.11. Optical Transmittance and Transmission Haze
3. Results and Discussion
3.1. Chemical Composition of Initial Pulps
3.2. Production of Cellulose Nanofibers: Properties of Nanofiber Suspensions
3.3. FTIR Analysis
3.4. Crystallinity of Cellulose Nanofibers
3.5. Thermal Stability of Cellulose Nanofibers
3.6. Nanopapers Properties
3.6.1. Mechanical Properties
3.6.2. Water Vapor Sorption Isotherms
3.6.3. Barrier Properties
3.6.4. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martín, J.A.; Sobrino-Plata, J.; Rodríguez-Calcerrada, J.; Collada, C.; Gil, L. Breeding and scientific advances in the fight against Dutch elm disease: Will they allow the use of elms in forest restoration? New For. 2019, 50, 183–215. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Pecori, F.; Pepori, A.L.; Ferrini, F.; Ghelardini, L. Genotype × environment interaction and growth stability of several elm clones resistant to Dutch elm disease. For. Ecol. Manag. 2010, 260, 1017–1025. [Google Scholar] [CrossRef]
- Martin, J.A.; Solla, A.; Venturas, M.; Collada, C.; Dominguez, J.; Miranda, E.; Fuentes, P.; Buron, M.; Iglesias, S.; Gil, L. Seven Ulmus minor clones tolerant to Ophiostoma novo-ulmi registered as forest reproductive material in Spain. iForest Biogeosci. For. 2015, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Arias, C.; Sobrino-Plata, J.; Macaya-Sanz, D.; Aguirre, N.M.; Collada, C.; Gil, L.; Martín, J.A.; Rodríguez-Calcerrada, J. Changes in plant function and root mycobiome caused by flood and drought in a riparian tree. Tree Physiol. 2020, 40, 886–903. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; Fillat, Ú.; Martín, J.A.; Aranda, P.; Ruiz-Hitzky, E.; Ibarra, D.; Wicklein, B. Biorefinery of Lignocellulosic Biomass from an Elm Clone: Production of Fermentable Sugars and Lignin-Derived Biochar for Energy and Environmental Applications. Energy Technol. 2019, 7, 277–287. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Lichtenstein, K.; Lavoine, N. Toward a deeper understanding of the thermal degradation mechanism of nanocellulose. Polym. Degrad. Stab. 2017, 146, 53–60. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, B.; Du, H.; Lv, D.; Zhang, Y.; Yu, G.; Mu, X.; Peng, H. Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr. Polym. 2016, 151, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Solala, I.; Volperts, A.; Andersone, A.; Dizhbite, T.; Mironova-Ulmane, N.; Vehniäinen, A.; Pere, J.; Vuorinen, T. Mechanoradical formation and its effects on birch kraft pulp during the preparation of nanofibrillated cellulose with Masuko refining. Holzforschung 2011, 66, 477–483. [Google Scholar] [CrossRef]
- NREL. TP-510-42618—Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Ferrer, A.; Quintana, E.; Filpponen, I.; Solala, I.; Vidal, V.; Rodríguez, A.; Laine, J.; Rojas, O.J. Effect of Residual Lignin and Heteropolysaccharides in Nanofibrillar Cellulose and Nanopaper. Cellulose 2012, 19, 2179–2193. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the potential of lignin-containing cellulose nanofibrils (LCNFs): A review on properties and applications. Cellulose 2020, 27, 1853–1877. [Google Scholar] [CrossRef]
- Martin-Sampedro, R.; Revilla, E.; Villar, J.C.; Eugenio, M.E. Enhancement of enzymatic saccharification of Eucalyptus globulus: Steam explosion versus steam treatment. Bioresour. Technol. 2014, 167. [Google Scholar] [CrossRef]
- Wollboldt, R.P.; Zuckerstätter, G.; Weber, H.K.; Larsson, P.T.; Sixta, H. Accessibility, reactivity and supramolecular structure of E. globulus pulps with reduced xylan content. Wood Sci. Technol. 2010, 44, 533–546. [Google Scholar] [CrossRef]
- Okita, Y.; Saito, T.; Isogai, A. TEMPO-mediated oxidation of softwood thermomechanical pulp. Holzforschung 2009, 63, 529–535. [Google Scholar] [CrossRef]
- Gharehkhani, S.; Sadeghinezhad, E.; Kazi, S.N.; Yarmand, H.; Badarudin, A.; Safaei, M.R.; Zubir, M.N.M. Basic effects of pulp refining on fiber properties—A review. Carbohydr. Polym. 2015, 115, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Larsson, P.T.; Dahlman, O. Distribution of Uronic Acids in Xylans from Various Species of Soft- and Hardwood As Determined by MALDI Mass Spectrometry. Biomacromolecules 2001, 2, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Saito, T.; Isogai, A. Entire Surface Oxidation of Various Cellulose Microfibrils by TEMPO-Mediated Oxidation. Biomacromolecules 2010, 11, 1696–1700. [Google Scholar] [CrossRef]
- Esteban, L.G.; Guindeo, A. Anatomía de las Maderas de Frondosas Españolas; AITIM: Madrid, Spain, 1990. [Google Scholar]
- Eyholzer, C.; Bordeanu, N.; Lopez-Suevos, F.; Rentsch, D.; Zimmermann, T.; Oksman, K. Preparation and characterization of water-redispersible nanofibrillated cellulose in powder form. Cellulose 2010, 17, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef]
- Sugiyama, J.; Persson, J.; Chanzy, H. Combined infrared and electron diffraction study of the polymorphism of native celluloses. Macromolecules 1991, 24, 2461–2466. [Google Scholar] [CrossRef]
- Fujisawa, S.; Okita, Y.; Fukuzumi, H.; Saito, T.; Isogai, A. Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr. Polym. 2011, 84, 579–583. [Google Scholar] [CrossRef]
- Fillat, Ú.; Wicklein, B.; Martín-Sampedro, R.; Ibarra, D.; Ruiz-Hitzky, E.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Reiner, R.R.; Ralph, S.A. Cellulose crystallinity of woods, wood pulps, and agricultural fibers by FT-Raman spectroscopy. In Proceedings of the 16th International Symposium on Wood, Fiber and Pulping Chemistry—Proceedings ISWFPC, Tianjin, China, 8–10 June 2011; Volume 1, pp. 69–74. [Google Scholar]
- Iwamoto, S.; Nakagaito, A.N.; Yano, H. Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl. Phys. A 2007, 89, 461–466. [Google Scholar] [CrossRef]
- Xu, H.; Li, B.; Mu, X.; Yu, G.; Liu, C.; Zhang, Y.; Wang, H. Quantitative characterization of the impact of pulp refining on enzymatic saccharification of the alkaline pretreated corn stover. Bioresour. Technol. 2014, 169, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, G.; Du, X.; Wang, Z.; Xu, S.; Zhang, Y. Extraction and characterization of cellulose nanofibers and nanocrystals from liquefied banana pseudo-stem residue. Compos. Part B 2019, 160, 341–347. [Google Scholar] [CrossRef]
- Imani, M.; Ghasemian, A.; Dehghani-Firouzabadi, M.R.; Afra, E.; Borghei, M.; Johansson, L.S.; Gane, P.A.C.; Rojas, O.J. Coupling Nanofibril Lateral Size and Residual Lignin to Tailor the Properties of Lignocellulose Films. Adv. Mater. Interfaces 2019, 6, 1900770. [Google Scholar] [CrossRef]
- Claro, F.C.; Matos, M.; Jordão, C.; Avelino, F.; Lomonaco, D.; Magalhães, W.L.E. Enhanced microfibrillated cellulose-based film by controlling the hemicellulose content and MFC rheology. Carbohydr. Polym. 2019, 218, 307–314. [Google Scholar] [CrossRef]
- Xu, J.; Krietemeyer, E.F.; Boddu, V.M.; Liu, S.X.; Liu, W.-C. Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover. Carbohydr. Polym. 2018, 192, 202–207. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Fukuzumi, H.; Saito, T.; Isogai, A. Influence of TEMPO-oxidized cellulose nanofibril length on film properties. Carbohydr. Polym. 2013, 93, 172–177. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Lebrun, L.; Khelifi, B.; Marais, S.; Dufresne, A. Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films. Carbohydr. Polym. 2011, 83, 1740–1748. [Google Scholar] [CrossRef]
- Meriçer, Ç.; Minelli, M.; Giacinti Baschetti, M.; Lindström, T. Water sorption in microfibrillated cellulose (MFC): The effect of temperature and pretreatment. Carbohydr. Polym. 2017, 174, 1201–1212. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Cunha, A.G.; Rojo, E.; Papageorgiou, A.C.; Rautkari, L.; Arboleda, J.C.; Rojas, O.J. Strength and Water Interactions of Cellulose I Filaments Wet-Spun from Cellulose Nanofibril Hydrogels. Sci. Rep. 2016, 6, 30695. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Hu, Y.; Wu, Y. Water vapor sorption properties of TEMPO oxidized and sulfuric acid treated cellulose nanocrystal films. Carbohydr. Polym. 2018, 197, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Österberg, M.; Vartiainen, J.; Lucenius, J.; Hippi, U.; Seppälä, J.; Serimaa, R.; Laine, J. A Fast Method to Produce Strong NFC Films as a Platform for Barrier and Functional Materials. ACS Appl. Mater. Interfaces 2013, 5, 4640–4647. [Google Scholar] [CrossRef]
- Jiang, F.; Li, T.; Li, Y.; Zhang, Y.; Gong, A.; Dai, J.; Hitz, E.; Luo, W.; Hu, L. Wood-Based Nanotechnologies toward Sustainability. Adv. Mater. 2018, 30, 1703453. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhu, H.; Bao, W.; Preston, C.; Liu, Z.; Dai, J.; Li, Y.; Hu, L. Highly transparent paper with tunable haze for green electronics. Energy Environ. Sci. 2014, 7, 3313–3319. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-Lignin Biodegradable and Flexible UV Protection Film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Koga, H.; Suganuma, K.; Nogi, M. Hazy Transparent Cellulose Nanopaper. Sci. Rep. 2017, 7, 41590. [Google Scholar] [CrossRef]






| Ethanol Extractives | Klason Lignin | Acid Soluble Lignin | Total Lignin | Glucan | Xylan | |
|---|---|---|---|---|---|---|
| UnBl-Elm | 1.3 ± 0.1 | 1.6 ± 0.1 | 0.9 ± 0.0 | 2.5 ± 0.1 | 75.4 ± 2.3 | 18.4 ± 0.5 |
| Bl-Elm | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.5 ± 0.1 | 1.4 ± 0.1 | 74.7 ± 1.8 | 17.0 ± 0.9 |
| Bl-Eu | 0.4 ± 0.2 | 0.6 ± 0.3 | 0.7 ± 0.1 | 1.3 ± 0.3 | 74.2 ± 0.9 | 19.3 ± 0.4 |
| Carbox. (µmol/g) | YNano (%) | T600 (%) | Z-Pot. (mV) | CrI (%) | CrS (nm) | |
|---|---|---|---|---|---|---|
| CNF-UnBl-Elm | 56 ± 4 | 50 ± 2 | 14 ± 2 | −7 ± 1 | 84 ± 1 | 8.2 ± 0.1 |
| CNF-Bl-Elm | 53 ± 9 | 61 ± 3 | 19 ± 1 | −28 ± 1 | 86 ± 3 | 9.8 ± 0.2 |
| CNF-Bl-Eu | 60 ± 7 | 45 ± 3 | 15 ± 2 | −10 ± 1 | 80 ± 6 | 9.2 ± 0.6 |
| TOCNF-Elm | 1178 ± 37 | 100 ± 0 | 93 ± 2 | −68 ± 1 | 84 ± 2 | 9.4 ± 0.2 |
| TOCNF-Eu | 1043 ± 150 | 100 ± 1 | 77 ± 3 | −68 ± 2 | 77 ± 2 | 8.2 ± 0.5 |
| Tdeg (°C) | Ton (°C) | Toff (°C) | ChR (%) | |
|---|---|---|---|---|
| CNF-UnBl-Elm | 353 | 316 | 373 | 33 |
| CNF-Bl-Elm | 350 | 310 | 370 | 26 |
| CNF-Bl-Eu | 347 | 312 | 369 | 18 |
| TOCNF-Elm | 241/295/396 | 220 | 476 | 32 |
| TOCNF-Eu | 240/291/399 | 222 | 484 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-López, L.; Eugenio, M.E.; Ibarra, D.; Darder, M.; Martín, J.A.; Martín-Sampedro, R. Cellulose Nanofibers from a Dutch Elm Disease-Resistant Ulmus minor Clone. Polymers 2020, 12, 2450. https://doi.org/10.3390/polym12112450
Jiménez-López L, Eugenio ME, Ibarra D, Darder M, Martín JA, Martín-Sampedro R. Cellulose Nanofibers from a Dutch Elm Disease-Resistant Ulmus minor Clone. Polymers. 2020; 12(11):2450. https://doi.org/10.3390/polym12112450
Chicago/Turabian StyleJiménez-López, Laura, María E. Eugenio, David Ibarra, Margarita Darder, Juan A. Martín, and Raquel Martín-Sampedro. 2020. "Cellulose Nanofibers from a Dutch Elm Disease-Resistant Ulmus minor Clone" Polymers 12, no. 11: 2450. https://doi.org/10.3390/polym12112450
APA StyleJiménez-López, L., Eugenio, M. E., Ibarra, D., Darder, M., Martín, J. A., & Martín-Sampedro, R. (2020). Cellulose Nanofibers from a Dutch Elm Disease-Resistant Ulmus minor Clone. Polymers, 12(11), 2450. https://doi.org/10.3390/polym12112450