Preparation and Characteristics of Wet-Spun Filament Made of Cellulose Nanofibrils with Different Chemical Compositions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
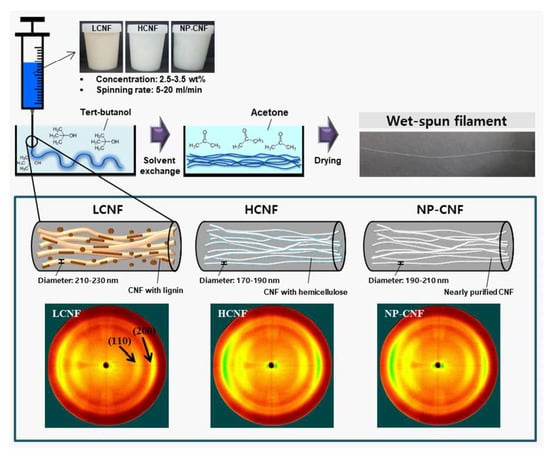
3.1. Chemical Composition
3.2. Morphological Characteristics
3.3. X-Ray Diffraction Anlaysis
3.4. Tensile Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abudula, T.; Saeed, U.; Memic, A.; Gauthaman, K.; Hussain, M.A.; Al-Turaif, H. Electrospun cellulose Nano fibril reinforced PLA/PBS composite scaffold for vascular tissue engineering. J. Polym. Res. 2019, 26, 110. [Google Scholar] [CrossRef]
- Endo, R.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose nanofibril/poly (vinyl alcohol) composite drawn fibers. Polymer 2013, 54, 935–941. [Google Scholar] [CrossRef]
- De Barros, R.D.R.O.; de Sousa Paredes, R.; Endo, T.; da Silva Bon, E.P.; Lee, S.H. Association of wet disk milling and ozonolysis as pretreatment for enzymatic saccharification of sugarcane bagasse and straw. Bioresour. Technol. 2013, 136, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Isogai, A.; Iwata, T. Structure and mechanical properties of wet-spun fibers made from natural cellulose nanofibers. Biomacromolecules 2011, 12, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Edwards, J.V.; Guo, Z.; Prevost, N.T.; Nam, S.; Wu, Q.; French, A.D.; Xu, F. Structural variations of cotton cellulose nanocrystals from deep eutectic solvent treatment: Micro and nano scale. Cellulose 2019, 26, 861–876. [Google Scholar] [CrossRef]
- Davoudpour, Y.; Hossain, M.S.; Abdul Khalil, H.P.S.; Mohamad Haafiz, M.K.; Mohd Ishak, Z.A.; Hassan, A.; Sarker, Z.I. Optimization of high pressure homogenization parameters for the isolation of cellulosic nanofibers using response surface methodology. Ind. Crop. Prod. 2015, 74, 381–387. [Google Scholar] [CrossRef]
- Park, C.W.; Han, S.Y.; Seo, P.N.; Youe, W.J.; Kim, Y.S.; Choi, S.K.; Kim, N.H.; Lee, S.H. Property comparison of thermoplastic starch reinforced by cellulose nanofibrils with different chemical compositions. BioResources 2019, 14, 1564–1578. [Google Scholar]
- Walther, A.; Timonen, J.V.I.; Díez, I.; Laukkanen, A.; Ikkala, O. Multifunctional high-performance biofibers based on wet-extrusion of renewable native cellulose nanofibrils. Adv. Mater. 2011, 23, 2924–2928. [Google Scholar] [CrossRef]
- Zheng, Q.; Cai, Z.; Gong, S. Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels—Scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A critical review of manufacturing processes used in regenerated cellulosic fibres: Viscose, cellulose acetate, cuprammonium, LiCl/DMAc, ionic liquids, and NMMO based lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Environmental impact assessment of man-made cellulose fibres. Resour. Conserv. Recycl. 2010, 55, 260–274. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Asaadi, S.; Ahvenainen, P.; Sixta, H. Water-induced crystallization and nano-scale spinodal decomposition of cellulose in NMMO and ionic liquid dope. Cellulose 2019, 26, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Collier, J.; Oyetunji, R.; Stutts, B.; Burnett, R. Enzymatic hydrolysis of cellulose dissolved in N-methyl morpholine oxide/water solutions. Bioresour. Technol. 2010, 101, 4965–4970. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, P.R.; Niklasson, C.; Taherzadeh, M.J. A pilot study on lignocelluloses to ethanol and fish feed using NMMO pretreatment and cultivation with zygomycetes in an air-lift reactor. Bioresour. Technol. 2011, 102, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Kafy, A.; Kim, H.C.; Zhai, L.; Kim, J.W.; Kang, T.J. Cellulose long fibers fabricated from cellulose nanofibers and its strong and tough characteristics. Sci. Rep. 2017, 7, 17683. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Cunha, A.G.; Rojo, E.; Papageorgiou, A.C.; Rautkari, L.; Arboleda, J.C.; Rojas, O.J. Strength and Water Interactions of Cellulose I Filaments Wet-Spun from Cellulose Nanofibril Hydrogels. Sci. Rep. 2016, 6, 30695. [Google Scholar] [CrossRef]
- Lundahl, M.J.; Klar, V.; Wang, L.; Ago, M.; Rojas, O.J. Spinning of cellulose nanofibrils into filaments: A review. Ind. Eng. Chem. Res. 2017, 56, 8–19. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Bordes, R.; Köhnke, T. Wet spinning of flame-retardant cellulosic fibers supported by interfacial complexation of cellulose nanofibrils with silica nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 39069–39077. [Google Scholar] [CrossRef]
- Park, C.W.; Han, S.Y.; Namgung, H.W.; Seo, P.N.; Lee, S.Y.; Lee, S.H. Preparation and characterization of cellulose nanofibrils with varying chemical compositions. BioResources 2017, 12, 5031–5044. [Google Scholar] [CrossRef] [Green Version]
- Park, C.W.; Han, S.Y.; Choi, S.K.; Lee, S.H. Preparation and properties of holocellulose nanofibrils with different hemicellulose content. BioResources 2017, 12, 6298–6308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tsuzuki, T.; Wang, X. Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 2015, 22, 1729–1741. [Google Scholar] [CrossRef]
- Kumagai, A.; Lee, S.H.; Endo, T. Evaluation of the effect of hot-compressed water treatment on enzymatic hydrolysis of lignocellulosic nanofibrils with different lignin content using a quartz crystal microbalance. Biotechnol. Bioeng. 2016, 113, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chang, F.; Inoue, S.; Endo, T. Increase in enzyme accessibility by generation of nanospace in cell wall supramolecular structure. Bioresour. Technol. 2010, 101, 7218–7223. [Google Scholar] [CrossRef]
- Galland, S.; Berthold, F.; Prakobna, K.; Berglund, L.A. Holocellulose Nanofibers of High Molar Mass and Small Diameter for High-Strength Nanopaper. Biomacromolecules 2015, 16, 2427–2435. [Google Scholar] [CrossRef]
- Prakobna, K.; Kisonen, V.; Xu, C.; Berglund, L.A. Strong reinforcing effects from galactoglucomannan hemicellulose on mechanical behavior of wet cellulose nanofiber gels. J. Mater. Sci. 2015, 50, 7413–7423. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Kim, J.C. Nanocellulose applications for drug delivery: A review. J. For. Environ. Sci. 2019, 35, 141–149. [Google Scholar]





| Chemical Composition (%) | Number of HPH Passes | Average Diameter (nm) | Specific Surface Area (m2/g) | Filtration Time (sec) | |||
|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Klason Lignin | |||||
| LCNF | 79.1 | 6.0 | 13.0 | 5 | 25.9 ± 11.4 | 142.8 | 259 |
| HCNF | 63.3 | 36.6 | - | 5 | 18.5 ± 3.1 | 182.1 | 394 |
| NP-CNF | 94.9 | 5.1 | - | 5 | 18.3 ± 2.4 | 180.5 | 321 |
| Concentration (wt.%) | Spinning Rate (mL/min) | Average Diameter (nm) | Density (g/cm3) | Orientation Index | |
|---|---|---|---|---|---|
| LCNF | 3.5 | 20 | 224 ± 15 | 0.73 ± 0.02 | 0.57 |
| HCNF | 2.5 | 5 | 175 ± 17 | 0.83 ± 0.04 | 0.64 |
| 10 | 151 ± 11 | 0.84 ± 0.03 | 0.66 | ||
| 20 | 143 ± 9 | 0.86 ± 0.01 | 0.67 | ||
| 3.5 | 20 | 181 ± 13 | 0.87 ± 0.05 | 0.60 | |
| NP-CNF | 3.5 | 20 | 199 ± 16 | 0.83 ± 0.03 | 0.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-W.; Park, J.-S.; Han, S.-Y.; Lee, E.-A.; Kwon, G.-J.; Seo, Y.-H.; Gwon, J.-G.; Lee, S.-Y.; Lee, S.-H. Preparation and Characteristics of Wet-Spun Filament Made of Cellulose Nanofibrils with Different Chemical Compositions. Polymers 2020, 12, 949. https://doi.org/10.3390/polym12040949
Park C-W, Park J-S, Han S-Y, Lee E-A, Kwon G-J, Seo Y-H, Gwon J-G, Lee S-Y, Lee S-H. Preparation and Characteristics of Wet-Spun Filament Made of Cellulose Nanofibrils with Different Chemical Compositions. Polymers. 2020; 12(4):949. https://doi.org/10.3390/polym12040949
Chicago/Turabian StylePark, Chan-Woo, Ji-Soo Park, Song-Yi Han, Eun-Ah Lee, Gu-Joong Kwon, Young-Ho Seo, Jae-Gyoung Gwon, Sun-Young Lee, and Seung-Hwan Lee. 2020. "Preparation and Characteristics of Wet-Spun Filament Made of Cellulose Nanofibrils with Different Chemical Compositions" Polymers 12, no. 4: 949. https://doi.org/10.3390/polym12040949
APA StylePark, C. -W., Park, J. -S., Han, S. -Y., Lee, E. -A., Kwon, G. -J., Seo, Y. -H., Gwon, J. -G., Lee, S. -Y., & Lee, S. -H. (2020). Preparation and Characteristics of Wet-Spun Filament Made of Cellulose Nanofibrils with Different Chemical Compositions. Polymers, 12(4), 949. https://doi.org/10.3390/polym12040949