Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals for Solution Preparation
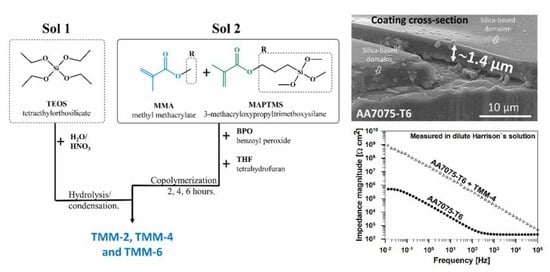
2.2. Synthesis of the Solution
2.3. Sample Preparation
2.4. Characterisation of the Synthesis
2.4.1. Fourier Transform Infrared Spectroscopy
2.4.2. Dynamic Light Scattering
2.4.3. Gel Permeation Chromatography
2.4.4. Thermal Analysis
2.4.5. Physico-Chemical Characterisation
2.5. Coating Characterisation
2.5.1. Morphology
2.5.2. Corrosion Performance
3. Results
3.1. Synthesis of the Sol-Gel Solution
3.1.1. Hydrolysis and Condensation of TEOS Studied by FTIR and DLS
3.1.2. The Copolymerization Process between MMA and MAPTMS, Studied by FTIR, DLS and GPC
3.1.3. The Mixing of Copolymerised Acrylates and Hydrolysed/Condensed TEOS Studied by FTIR, DLS and GPC
3.2. Solution Characterisation
3.2.1. Sol Density and Viscosity
3.2.2. Thermal Analysis
3.3. Coating Characterisation
3.3.1. Morphology, Composition and Thickness
3.3.2. Corrosion Performance
4. Conclusions
- FTIR and DLS confirmed the formation of silica (Si–O–Si) domains from TEOS during hydrolysis/condensation reactions.
- FTIR, DLS and GPC confirmed that copolymerization between MMA and MAPTMS is time-dependent; the process was not complete even after 6 h.
- The structure of partially polymerised copolymers had a crucial effect on the subsequent hydrolysis/condensation reactions during formation of the organosilane matrix.
- The viscosity, and consequently the thickness, of the TMM coatings on AA7075-T6 also depended on the copolymerization time.
- The results of TG and DSC confirm that the optimal curing temperature is 166 °C.
- The FESEM/EDS established the presence of randomly spread silica-rich domains in the coating.
- The EIS revealed that the highest impedance was obtained with TMM-4 and TMM-6 coatings, confirming the importance of copolymerization time.
- Despite only a few-micrometre-thick coating, TMM-4 possessed high barrier properties and durability for more than four months in dilute Harrison’s solution.
- The TMM-4 coating exhibited the most substantial barrier properties, due to the more favourable copolymerised structure.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A




References
- Singh, S.S.; Guo, E.; Xie, H.; Chawla, N. Mechanical properties of intermetallic inclusions in Al 7075 alloys by micropillar compression. Intermetallics 2015, 62, 69–75. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 1980–2015 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Zhao, Z.; Frankel, G.S. On the first breakdown in AA7075-T6. Corros. Sci. 2007, 49, 3064–3088. [Google Scholar] [CrossRef]
- Zhao, Z.; Frankel, G.S. The effect of temper on the first breakdown in AA7075. Corros. Sci. 2007, 49, 3089–3111. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J.H.W. Corrosion behaviour of different tempers of AA7075 aluminium alloy. Electrochimica Acta 2004, 49, 2851–2862. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Corrosion Inhibition of Pure Aluminium and Alloys AA2024-T3 and AA7075-T6 by Cerium(III) and Cerium(IV) Salts. J. Electrochem. Soc. 2016, 163, C85–C93. [Google Scholar] [CrossRef]
- Milošev, I.; Rodič, P. Cerium chloride and acetate salts as corrosion inhibitors for aluminium alloy AA7075-T6 in sodium chloride solution. Corrosion 2016, 72, 1021–1034. [Google Scholar] [CrossRef]
- Andreatta, F.; Lohrengel, M.M.; Terryn, H.; de Wit, J.H.W. Electrochemical characterisation of aluminium AA7075-T6 and solution heat treated AA7075 using a micro-capillary cell. Electrochimica Acta 2003, 48, 3239–3247. [Google Scholar] [CrossRef]
- Souto, R.M.; González, S.; Fernández-Pérez, B.M.; Pérez, B.M.F. A novel approach to the mathematical modelling of atmospheric corrosion rates from fragmented subtropical environments. Eur. J. Sci. Theol. 2015, 11, 241–252. [Google Scholar]
- Environments, A.S.; Hernández, F.J.; Santana, J.J.; Souto, R.M.; González, S.; Morales, J. Characterization of the Atmospheric Corrosion of Aluminum in Archipelagic Subtropical Environments. Int. J. Electrochem. Sci. 2011, 6, 6567–6580. [Google Scholar]
- Huang, I.-W.; Hurley, B.L.; Yang, F.; Buchheit, R.G. Dependence on Temperature, pH, and Cl− in the Uniform Corrosion of Aluminum Alloys 2024-T3, 6061-T6, and 7075-T6. Electrochimica Acta 2016, 199, 242–253. [Google Scholar] [CrossRef]
- Knight, S.P.; Salagaras, M.; Trueman, A.R. The study of intergranular corrosion in aircraft aluminium alloys using X-ray tomography. Corros. Sci. 2011, 53, 727–734. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Corrosion and Corrosion Protection of Aluminum Alloys. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 115–127. ISBN 978-0-12-809894-3. [Google Scholar]
- Abdolah Zadeh, M.; Van der Zwaag, S.; Garcia, S.J. Routes to extrinsic and intrinsic self-healing corrosion protective sol-gel coatings: a review. Self-Heal. Mater. 2013, 1, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Stankiewicz, A.; Szczygieł, I.; Szczygieł, B. Self-healing coatings in anti-corrosion applications. J. Mater. Sci. 2013, 48, 8041–8051. [Google Scholar] [CrossRef] [Green Version]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Skeldon, P.; Thompson, G.E.; Habazaki, H.; Shimizu, K. Chromate conversion coatings on aluminium: influences of alloying. Corros. Sci. 2004, 46, 297–312. [Google Scholar] [CrossRef]
- Qi, J.-T.; Hashimoto, T.; Walton, J.R.; Zhou, X.; Skeldon, P.; Thompson, G.E. Trivalent chromium conversion coating formation on aluminium. Surf. Coat. Technol. 2015, 280, 317–329. [Google Scholar] [CrossRef]
- Langård, S. Biological and Environmental Aspects of Chromium; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1-4832-9018-8. [Google Scholar]
- Park, R.M.; Bena, J.F.; Stayner, L.T.; Smith, R.J.; Gibb, H.J.; Lees, P.S.J. Hexavalent Chromium and Lung Cancer in the Chromate Industry: A Quantitative Risk Assessment. Risk Anal. 2004, 24, 1099–1108. [Google Scholar] [CrossRef]
- Baldwin, K.R.; Smith, C.J.E. Accelerated corrosion tests for aerospace materials: current limitations and future trends. Aircr. Eng. Aerosp. Technol. 1999, 71, 239–244. [Google Scholar] [CrossRef]
- Twite, R.; Bierwagen, G. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Prog. Org. Coat. 1998, 33, 91–100. [Google Scholar] [CrossRef]
- Carreira, A.F.; Pereira, A.M.; Vaz, E.P.; Cabral, A.M.; Ghidini, T.; Pigliaru, L.; Rohr, T. Alternative corrosion protection pretreatments for aluminum alloys. J. Coat. Technol. Res. 2017, 14, 879–892. [Google Scholar] [CrossRef] [Green Version]
- Kunst, S.R.; Ludwig, G.A.; Cardoso, H.R.P.; Santana, J.A.; Sarmento, V.H.V.; de Fraga Malfatti, C. Hybrid films with (trimethoxysilylpropyl) methacrylate (TMSM), poly (methyl methacrylate) PMMA and tetraethoxysilane (TEOS) applied on tinplate. Mater. Res. 2014, 17, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, R.V.; Bharathidasan, T.; Basu, B.J. Superhydrophobic sol-gel nanocomposite coatings with enhanced hardness. Appl. Surf. Sci. 2011, 257, 10421–10426. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; Torrico, R.F.O.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Organic-Inorganic Hybrid Coatings for Corrosion Protection of Metallic Surfaces. In New Technologies in Protective Coatings; Giudice, C., Canosa, G., Eds.; InTech: London, UK, 2017; ISBN 978-953-51-3491-6. [Google Scholar]
- Kunst, S.R.; Cardoso, H.R.P.; Oliveira, C.T. Influence of Tetraethoxysilane Addition in Siloxane- Poly(Methyl Methacrylate) Hybrid Films Applied on Galvanized Steel. Int J Electrochem Sci 2013, 8, 21. [Google Scholar]
- dos Santos, F.C.; Harb, S.V.; Menu, M.-J.; Turq, V.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. On the structure of high performance anticorrosive PMMA–siloxane–silica hybrid coatings. RSC Adv. 2015, 5, 106754–106763. [Google Scholar] [CrossRef] [Green Version]
- Poberžnik, M.; Costa, D.; Hemeryck, A.; Kokalj, A. Insight into the Bonding of Silanols to Oxidized Aluminum Surfaces. J. Phys. Chem. C 2018, 122, 9417–9431. [Google Scholar] [CrossRef]
- Poberžnik, M.; Kokalj, A. Implausibility of bidentate bonding of the silanol headgroup to oxidized aluminum surfaces. Appl. Surf. Sci. 2019, 492, 909–918. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Pellice, S.A.; Castro, Y.; Aparicio, M.; Durán, A. Improved corrosion resistance of AA2024 alloys through hybrid organic–inorganic sol–gel coatings produced from sols with controlled polymerisation. Surf. Coat. Technol. 2009, 203, 1897–1903. [Google Scholar] [CrossRef]
- Mosa, J.; Rosero-Navarro, N.C.; Aparicio, M. Active corrosion inhibition of mild steel by environmentally-friendly Ce-doped organic–inorganic sol–gel coatings. RSC Adv. 2016, 6, 39577–39586. [Google Scholar] [CrossRef]
- Sakai, R.T.; da Cruz, F.M.D.L.; de Melo, H.G.; Benedetti, A.V.; Santilli, C.V.; Suegama, P.H. Electrochemical study of TEOS, TEOS/MPTS, MPTS/MMA and TEOS/MPTS/MMA films on tin coated steel in 3.5% NaCl solution. Prog. Org. Coat. 2012, 74, 288–301. [Google Scholar] [CrossRef]
- Studer, K.; Decker, C.; Beck, E.; Schwalm, R. Overcoming oxygen inhibition in UV-curing of acrylate coatings by carbon dioxide inerting, Part I. Prog. Org. Coat. 2003, 48, 92–100. [Google Scholar] [CrossRef]
- Studer, K.; Decker, C.; Beck, E.; Schwalm, R. Overcoming oxygen inhibition in UV-curing of acrylate coatings by carbon dioxide inerting: Part II. Prog. Org. Coat. 2003, 48, 101–111. [Google Scholar] [CrossRef]
- Hamulić, D.; Rodič, P.; Poberžnik, M.; Jereb, M.; Kovač, J.; Milošev, I. The Effect of the Methyl and Ethyl Group of the Acrylate Precursor in Hybrid Silane Coatings Used for Corrosion Protection of Aluminium Alloy 7075-T6. Coatings 2020, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Rodič, P.; Lekka, M.; Andreatta, F.; Fedrizzi, L.; Milošev, I. The effect of copolymerization on the performance of acrylate-based hybrid sol-gel coating for corrosion protection of AA2024-T3. Prog. Org. Coat. in press.
- Trentin, A.; Gasparini, A.d.L.; Faria, F.A.; Harb, S.V.; dos Santos, F.C.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Barrier properties of high performance PMMA-silica anticorrosion coatings. Prog. Org. Coat. 2020, 138, 105398. [Google Scholar] [CrossRef]
- Harb, S.V.; Trentin, A.; de Souza, T.A.C.; Magnani, M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Effective corrosion protection by eco-friendly self-healing PMMA-cerium oxide coatings. Chem. Eng. J. 2019, 123219, in press. [Google Scholar] [CrossRef]
- Kunst, S.R.; dos Santos, R.F.; Vieira, D.; Oliveira, C.T.; Sarmento, V.H.V.; Malfatti, C.d.F. Elaboration and Characterization of Siloxane-PMMA Hybrid Films on Tinplate Substrates. Mater. Sci. Forum 2012, 1610–1615. [Google Scholar] [CrossRef]
- Miyazaki, K.; Takata, T.; Endo, T.; Inanaga, A. Thermal- and photo-polymerization of (meth) acrylates containing a spiro ortho ester moiety and the properties of poly[(meth)acrylate]s. Dent. Mater. J. 1994, 13, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Su, W.-F. Radical Chain Polymerization. In Principles of Polymer Design and Synthesis; Su, W.-F., Ed.; Lecture Notes in Chemistry; Springer: Berlin, Heidelberg, Germany, 2013; pp. 137–183. ISBN 978-3-642-38730-2. [Google Scholar]
- Huang, N.-J.; Sundberg, D.C. Fundamental studies of grafting reactions in free radical copolymerization. II. Grafting of styrene, acrylate, and methacrylate monomers onto cis-polybutadiene using AIBN initiator in solution polymerization. J. Polym. Sci. Part Polym. Chem. 1995, 33, 2551–2570. [Google Scholar] [CrossRef]
- Bauer, F.; Decker, U.; Naumov, S.; Riedel, C. Photoinitiator-free UV curing and matting of acrylate-based nanocomposite coatings: Part 3. Prog. Org. Coat. 2014, 77, 1085–1094. [Google Scholar] [CrossRef]
- Harb, S.V.; Cerrutti, B.M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Siloxane–PMMA hybrid anti-corrosion coatings reinforced by lignin. Surf. Coat. Technol. 2015, 275, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Harb, S.V.; Pulcinelli, S.H.; Santilli, C.V.; Knowles, K.M.; Hammer, P. A Comparative Study on Graphene Oxide and Carbon Nanotube Reinforcement of PMMA-Siloxane-Silica Anticorrosive Coatings. ACS Appl. Mater. Interfaces 2016, 8, 16339–16350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trentin, A.; Harb, S.V.; Uvida, M.C.; Pulcinelli, S.H.; Santilli, C.V.; Marcoen, K.; Pletincx, S.; Terryn, H.; Hauffman, T.; Hammer, P. Dual Role of Lithium on the Structure and Self-Healing Ability of PMMA-Silica Coatings on AA7075 Alloy. ACS Appl. Mater. Interfaces 2019, 11, 40629–40641. [Google Scholar] [CrossRef] [PubMed]
- Pellice, S.; Galliano, P.; Castro, Y.; Durán, A. Hybrid Sol-Gel Coatings Produced from TEOS and γ-MPS. J. Sol-Gel Sci. Technol. 2003, 28, 81–86. [Google Scholar] [CrossRef]
- Andreatta, F.; Paussa, L.; Lanzutti, A.; Rosero Navarro, N.C.; Aparicio, M.; Castro, Y.; Duran, A.; Ondratschek, D.; Fedrizzi, L. Development and industrial scale-up of ZrO2 coatings and hybrid organic–inorganic coatings used as pre-treatments before painting aluminium alloys. Prog. Org. Coat. 2011, 72, 3–14. [Google Scholar] [CrossRef]
- Andreatta, F.; Aldighieri, P.; Paussa, L.; Di Maggio, R.; Rossi, S.; Fedrizzi, L. Electrochemical behaviour of ZrO2 sol–gel pre-treatments on AA6060 aluminium alloy. Electrochimica Acta 2007, 52, 7545–7555. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I.; Lekka, M.; Andreatta, F.; Fedrizzi, L. Corrosion behaviour and chemical stability of transparent hybrid sol-gel coatings deposited on aluminium in acidic and alkaline solutions. Prog. Org. Coat. 2018, 124, 286–295. [Google Scholar] [CrossRef]
- Rodič, P.; Katić, J.; Korte, D.; Desimone, P.M.; Franko, M.; Ceré, S.M.; Metikoš-Huković, M.; Milošev, I. The Effect of Cerium Ions on the Structure, Porosity and Electrochemical Properties of Si/Zr-Based Hybrid Sol-Gel Coatings Deposited on Aluminum. Metals 2018, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Paussa, L.; Rosero-Navarro, N.C.; Andreatta, F.; Castro, Y.; Duran, A.; Aparicio, M.; Fedrizzi, L. Inhibition effect of cerium in hybrid sol–gel films on aluminium alloy AA2024. Surf. Interface Anal. 2010, 42, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Rosero-Navarro, N.C.; Figiel, P.; Jedrzejewski, R.; Biedunkiewicz, A.; Castro, Y.; Aparicio, M.; Pellice, S.A.; Durán, A. Influence of cerium concentration on the structure and properties of silica-methacrylate sol–gel coatings. J. Sol-Gel Sci. Technol. 2010, 54, 301–311. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Pellice, S.A.; Durán, A.; Ceré, S.; Aparicio, M. Corrosion protection of aluminium alloy AA2024 with cerium doped methacrylate-silica coatings. J. Sol-Gel Sci. Technol. 2009, 52, 31–40. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Harrison, J.B.; Tickle, T.C.K. Harrison solution. 1 Oil Color Chem. 1962, 45, 571. [Google Scholar]
- Rodič, P.; Iskra, J.; Milošev, I. A hybrid organic–inorganic sol–gel coating for protecting aluminium alloy 7075-T6 against corrosion in Harrison’s solution. J. Sol-Gel Sci. Technol. 2014, 70, 90–103. [Google Scholar] [CrossRef]
- Kesmez, Ö.; Burunkaya, E.; Kiraz, N.; Çamurlu, H.E.; Asiltürk, M.; Arpaç, E. Effect of acid, water and alcohol ratios on sol-gel preparation of antireflective amorphous SiO2 coatings. J. Non-Cryst. Solids 2011, 357, 3130–3135. [Google Scholar] [CrossRef]
- Hristov, D.R.; Mahon, E.; Dawson, K.A. Controlling aqueous silica nanoparticle synthesis in the 10–100 nm range. Chem. Commun. 2015, 51, 17420–17423. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non-Cryst. Solids 1988, 100, 31–50. [Google Scholar] [CrossRef] [Green Version]
- Battocchi, D.; Simões, A.M.; Tallman, D.E.; Bierwagen, G.P. Comparison of testing solutions on the protection of Al-alloys using a Mg-rich primer. Corros. Sci. 2006, 48, 2226–2240. [Google Scholar] [CrossRef]
- Joshi, S.; Fahrenholtz, W.G.; O’Keefe, M.J. Electrochemical Characterization of Al 7075-T6 Surface Oxide After Alkaline Treatments. J. Electrochem. Soc. 2011, 158, C296–C301. [Google Scholar] [CrossRef]
- Rodič, P.; Milošev, I. Corrosion Properties of UV Cured Hybrid Sol-Gel Coatings on AA7075-T6 Determined under Simulated Aircraft Conditions. J. Electrochem. Soc. 2014, 161, C412–C420. [Google Scholar] [CrossRef]
- McIntyre, J.M.; Pham, H.Q. Electrochemical impedance spectroscopy; a tool for organic coatings optimizations. Prog. Org. Coat. 1996, 27, 201–207. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and its Applications. In Modern Aspects of Electrochemistry; Modern Aspects of Electrochemistry; Springer: Boston, MA, USA, 2002; pp. 143–248. ISBN 978-0-306-45964-1. [Google Scholar]
- Bierwagen, G.P.; He, L.; Li, J.; Ellingson, L.; Tallman, D.E. Studies of a new accelerated evaluation method for coating corrosion resistance — thermal cycling testing. Prog. Org. Coat. 2000, 39, 67–78. [Google Scholar] [CrossRef]












| Sol | Mw [g/mol] | Mn [g/mol] | PDI |
|---|---|---|---|
| MMA/MAPTMS-prior | 8724 | 6252 | 1.40 |
| MMA/MAPTMS-2 h | 81166 | 14881 | 5.08 |
| MMA/MAPTMS-4 h | 83333 | 16483 | 5.06 |
| MMA/MAPTMS-6 h | 147562 | 18737 | 7.88 |
| TMM-2 | 79073 | 22215 | 3.56 |
| TMM-4 | 79303 | 21149 | 3.75 |
| TMM-6 | 81039 | 20097 | 4.03 |
| Sol | γ [g/mL] | ν [St] | μ [mPa s] | Coating Thickness [µm] |
|---|---|---|---|---|
| TMM-2 | 0.924563 | 2.68 ± 0.01 | 2.48 | 0.8 ± 0.2 |
| TMM-4 | 0.924371 | 3.39 ± 0.01 | 3.31 | 1.4 ± 0.2 |
| TMM-6 | 0.924616 | 4.21 ± 0.01 | 3.89 | 2.6 ± 0.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodič, P.; Korošec, R.C.; Kapun, B.; Mertelj, A.; Milošev, I. Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time. Polymers 2020, 12, 948. https://doi.org/10.3390/polym12040948
Rodič P, Korošec RC, Kapun B, Mertelj A, Milošev I. Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time. Polymers. 2020; 12(4):948. https://doi.org/10.3390/polym12040948
Chicago/Turabian StyleRodič, Peter, Romana Cerc Korošec, Barbara Kapun, Alenka Mertelj, and Ingrid Milošev. 2020. "Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time" Polymers 12, no. 4: 948. https://doi.org/10.3390/polym12040948
APA StyleRodič, P., Korošec, R. C., Kapun, B., Mertelj, A., & Milošev, I. (2020). Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time. Polymers, 12(4), 948. https://doi.org/10.3390/polym12040948