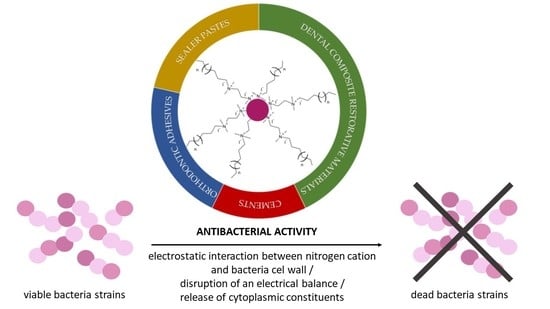
Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials
Abstract
:1. Introduction
2. Synthesis of QA-PEI Nanoparticles
- 1.
- Crosslinking: The goal of this process is to form PEI nanoparticles that consist of a crosslinked core with a large number of free-standing side chains. Due to the covalent bonding of the side chains, they represent an integral part of the core and cannot be dissociated [81]. The overall structure of a PEI nanoparticle contains primary, secondary, and tertiary amino groups. Primary amino groups are located at the ends of side chains, secondary amino groups are located within the core and the side chains, and tertiary amino groups are only located in the core. The difference between the two crosslinking methods is that, in the reductive amination process, glutaraldehyde is used as a crosslinking agent, whereas in the N-alkylation process, crosslinking occurs using an alkyl dihalide, usually 1,5-dibromopentane (Figure 2) [60]. 1,4-dibromobutane and 1,6-dibromohexane can also be used as suitable crosslinking agents [82].
- 2.
- Telomerization: This step aims to extend the side chains by adding long linear alkyl chains, which are crucial for defining the hydrophobic character of the nanoparticles. In fact, hydrophobicity governs the interactions of QA-PEI nanoparticles with lipids located in bacterial cell walls. The telomerization process involves the transformation of primary amino groups into secondary amino groups. Alkyl bromide is often used for this purpose in the N-alkylation method (Figure 3), whereas octanal is used in the reductive amination method (Figure 4).
- 3.
- Quaternization: This process results in the generation of quaternary ammonium groups. Specifically, the procedure targets secondary and tertiary amino groups that can react with an alkyl halide, which functions as the quaternization agent. Since iodomethane is typically used for this purpose, this step is also called N-methylation (Figure 5) [60]. The quaternization of amine groups is responsible for instilling the QA-PEI nanoparticles with their antibacterial properties. This stage is also crucial for determining the intensity of their antibacterial activity [60,85,86].
3. Antibacterial Properties of QA-PEI Nanoparticles
3.1. Crosslink Density
3.2. Length of N-alkyl Telomers
3.3. Degree of N-Alkylation
3.4. Degree of Quaternization
3.5. Effect of Counterion
3.6. Relationship Between the pH and Antibacterial Properties of QA-PEI Nanoparticles
3.7. Effect of Oxidizing Environment
4. Properties of Dental Composite Restorative Materials Modified with QA-PEI Nanoparticles
5. QA-PEI Nanoparticles in Other Dental Materials
6. Biocompatibility of Dental Materials Modified with QA-PEI Nanoparticles
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Watts, D.C. Dental Restorative Materials. In Materials Science and Technology: A Comprehensive Treatment; Cahn, R.W., Haasen, P., Kramer, E.J., Eds.; VCH: New York, NY, USA, 1992; Volume 14, pp. 209–258. [Google Scholar]
- Dursun, E.; Fron-Chabouis, H.; Attal, J.P.; Raskin, A. Bisphenol A Release: Survey of the Composition of Dental Composite Resins. Open Dent. J. 2016, 10, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in Vitro Quantitative Antibacterial Analysis of Amalgam and Composite Resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef]
- Padovani, G.C.; Fùcio, S.B.P.; Ambrosano, G.M.B.; Correr-Sobrinho, L.; Puppin-Rontani, R.M. In Situ Bacterial Accumulation on Dental Restorative Materials. CLSM/COMSTAT Analysis. Am. J. Dent. 2015, 28, 3–8. [Google Scholar]
- Ali, S.; Sangi, L.; Kumar, N.; Khurshid, Z.; Zafar, M.S. Evaluating antibacterial and surface mechanical properties of chitosan modified dental resin composites. Technol. Health. Care. 2020, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kleverlaan, C.J.; Feilzer, A.J. Polymerization Shrinkage and Contraction Stress of Dental Resin Composites. Dent. Mater. 2005, 21, 1150–1157. [Google Scholar] [CrossRef]
- El-Banna, A.; Sherief, D.; Fawzy, A.S. Resin-Based Dental Composites for Tooth Filling In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–173. [Google Scholar] [CrossRef]
- Lin, G.S.S.; Abdul Ghani, N.R.N.; Ismail, N.H.; Singbal, K.P.; Yusuff, N.M.M. Polymerization Shrinkage and Degree of Conversion of New Zirconia-Reinforced Rice Husk Nanohybrid Composite. Eur. J. Dent. 2020, 14, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Chisini, L.A.; Collares, K.; Cademartori, M.G.; de Oliveira, L.J.C.; Conde, M.C.M.; Demarco, F.F.; Corrêa, M.B. Restorations in Primary Teeth: A Systematic Review on Survival and Reasons for Failures. Int. J. Paediatr. Dent. 2018, 28, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Luis, H.; Martin, M.D.; Leroux, B.G.; Rue, T.; Leitão, J.; DeRouen, T.A. Survival and Reasons for Failure of Amalgam Versus Composite Posterior Restorations Placed in a Randomized Clinical Trial. J. Am. Dent. Assoc. 2007, 138, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M.; Montiel-Company, J.M. Antibacterial Properties of Nanoparticles in Dental Restorative Materials. A Systematic Review and Meta-Analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hua, H.; Li, W.; Wang, R.; Jiang, X.; Zhu, M. Strong antibacterial dental resin composites containing cellulose nanocrystal/zinc oxide nanohybrids. J. Dent. 2019, 80, 23–29. [Google Scholar] [CrossRef]
- Dias, H.B.; Bernardi, M.I.B.; Bauab, T.M.; Hernandes, A.C.; de Souza Rastelli, A.N. Titanium dioxide and modified titanium dioxide by silver nanoparticles as an antibiofilm filler content for composite resins. Dent. Mater. 2019, 35, e36–e46. [Google Scholar] [CrossRef] [PubMed]
- Florez, F.L.E.; Hiers, R.D.; Larson, P.; Johnson, M.; O’Rear, E.; Rondinone, A.J.; Khajotia, S.S. Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles. Mater. Sci. Eng. C 2018, 93, 931–943. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Zhou, X.; Weir, M.D.; Melo, M.A.S.; Tay, F.R.; Imazato, S.; Oates, T.W.; Cheng, L.; Xu, H.K.K. In vitro evaluation of composite containing DMAHDM and calcium phosphate nanoparticles on recurrent caries inhibition at bovine enamel-restoration margins. Dent. Mater. 2020, 36, 1343–1355. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Ruan, J.; Arola, D.; Ji, C.; Weir, M.; Oates, T.; Chang, X.; Zhang, K.; Xu, H. Bonding durability, antibacterial activity and biofilm pH of novel adhesive containing antibacterial monomer and nanoparticles of amorphous calcium phosphate. J. Dent. 2019, 81, 91–101. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafar, M.S.; Alnazzawi, A.A.; Alrahabi, M.; Fareed, M.A.; Najeeb, S.; Khurshid, Z. Nanotechnology and Nanomaterials in Dentistry In Advanced Dental Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 477–505. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Dharmadhikari, S.; Abdulla, A.M.; Bapat, P.; Alexander, A.; Dubey, S.K.; Kesharwani, P. Recent advances of gold nanoparticles as biomaterial in Dentistry. Int. J. Pharm. 2020, 583, 119596. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Gloria, A.; De Santis, R.; D’Amora, U.; Balato, G.; Vollaro, A.; Oliviero, O.; Improta, G.; Triassi, M.; Ambrosio, L. Preliminary Focus on the Mechanical and Antibacterial Activity of a PMMA-Based Bone Cement Loaded with Gold Nanoparticles. Bioact. Mater. 2017, 2, 156–161. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, M.; Lee, D.; Han, S.; Moon, J.H.; Lim, H.N.; Kwon, I.K. Preparation and characterization of antibacterial orthodontic resin containing silver nanoparticles. Appl. Surf. Sci. 2018, 432, 317–323. [Google Scholar] [CrossRef]
- Dutra-Correa, M.; Leite, A.A.B.V.; de Cara, S.P.H.M.; Diniz, I.M.A.; Marques, M.M.; Suffredini, I.B.; Fernandes, M.S.; Toma, S.H.; Araki, K.; Medeiros, I.S. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef]
- Zhang, R.; Jones, M.M.; Moussa, H.; Keskar, M.; Huo, N.; Zhang, Z.; Visser, M.B.; Sabatini, C.; Swihart, M.T.; Cheng, C. Polymer–Antibiotic Conjugates as Antibacterial Additives in Dental Resins. Biomater. Sci. 2018, 7, 287–295. [Google Scholar] [CrossRef]
- Boaro, L.C.C.; Campos, L.M.; Varca, G.H.C.; Dos Santos, T.M.R.; Marques, P.A.; Sugii, M.M.; Saldanha, N.R.; Cogo-Müller, K.; Brandt, W.C.; Braga, R.R.; et al. Antibacterial resin-based composite containing chlorhexidine for dental applications. Dent. Mater. 2019, 35, 909–918. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Liang, J.; Li, M.; Ren, B.; Wu, T.; Xu, H.H.K.; Liu, Y.; Peng, X.; Yang, G.; Weir, M.; Zhang, S.; et al. The anti-caries effects of dental adhesive resin influenced by the position of functional groups in quaternary ammonium monomers. Dent. Mater. 2018, 34, 400–411. [Google Scholar] [CrossRef]
- Ballhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Concentration dependence of quaternary ammonium monomer on the design of high-performance bioactive composite for root caries restorations. Dent. Mater. 2020, 36, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.C.; Mangabeira, A.; Vieira, R.; Neves, A.; Lopes, R.T.; Pires, T.M.; Viana, G.; Cabral, L.; Cavalcante, L.; Portela, M.B. Experimental composites containing quaternary ammonium methacrylates reduce demineralization at enamel-restoration margins after cariogenic challenge. Dent. Mater. 2019, 35, e175–e183. [Google Scholar] [CrossRef]
- Payne, M.E.; Lou, Y.; Zhang, X.; Sahiner, N.; Sandoval, N.R.; Shantz, D.F.; Grayson, S.M. Comparison of Cross-Linked Branched and Linear Poly(ethylene imine) Microgel Microstructures and Their Impact in Antimicrobial Behavior, Copper Chelation, and Carbon Dioxide Capture. ACS Appl. Polym. Mater. 2020, 2, 826–836. [Google Scholar] [CrossRef]
- Shen, C.; Li, J.; Zhang, Y.; Li, Y.; Shen, G.; Zhu, J.; Tao, J. Polyethylenimine-Based Micro/Nanoparticles as Vaccine Adjuvants. Int. J. Nanomed. 2017, 12, 5443–5460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lungu, C.N.; Diudea, M.V.; Putz, M.V.; Grudziński, I.P. Linear and Branched PEIs (Polyethylenimines) and Their Property Space. Int. J. Mol. Sci. 2016, 17, 555. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Z.; Sun, Y.; Chi, Z.; Qing, G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef]
- Song, J.; Wang, D.; Wang, J.; Shen, Q.; Xie, C.; Lu, W.; Wang, R.; Liu, M. Low molecular weight polyethyleneimine modified by 2-aminoimidazole achieving excellent gene transfection efficiency. Eur. Polym. 2020, 140, 110017. [Google Scholar] [CrossRef]
- Firoozi, B.; Nasser, Z.; Sofalian, O.; Sheikhzade-Mosadegh, P. Enhancement of the transfection efficiency of DNA into Crocus sativus L. cells via PEI nanoparticles. J. Integr. Agric. 2018, 17, 1768–1778. [Google Scholar] [CrossRef]
- Garg, C.; Sharma, A.K.; Gupta, A.; Kumar, P. Anisamido-Polyethylenimines as Efficient Nonviral Vectors for the Transport of Plasmid DNA to Sigma Receptor–Bearing Cells In Vitro. J. Pharm. Sci. 2019, 108, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, X.; Zhu, Y. Studies on the Preparation and Antibacterial Properties of Quaternized Polyethyleneimine. J. Biomater. Sci. Polym. Ed. 2007, 18, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Vakurov, A.; Simpson, C.E.; Daly, C.L.; Gibson, T.D.; Millner, P.A. Acetylcholinesterase-Based Biosensor Electrodes for Organophosphate Pesticide Detection. I. Modification of Carbon Surface for Immobilization of Acetylcholinesterase. Biosens. Bioelectron. 2004, 20, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Pessela, B.C.C.; Fernández-Lafuente, R.; Fuentes, M.; Vián, A.; Garcı́a, J.L.; Carrascosa, A.V.; Mateo, C.; Guisán, J.M. Reversible Immobilization of a Thermophilic β-Galactosidase via Ionic Adsorption on PEI-Coated Sepabeads. Enzyme Microb. Tech. 2003, 32, 369–374. [Google Scholar] [CrossRef]
- De Rosa, G.; Bochot, A.; Quaglia, F.; Besnard, M.; Fattal, E. A New Delivery System for Antisense Therapy: PLGA Microspheres Encapsulating Oligonucleotide/Polyethyleneimine Solid Complexes. Int. J. Pharm. 2003, 254, 89–93. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Ren, T.; Jia, C.; Yang, Z.; Sun, H. Folate-modified carboxymethyl-chitosan/polyethylenimine/bovine serum albumin based complexes for tumor site-specific drug delivery. Carbohydr. Polym. 2018, 198, 76–85. [Google Scholar] [CrossRef]
- Liang, L.; Chen, Y.; Chen, X.M.; Zhang, Y.; Liu, Y. Cyclodextrin/polyethylenimine-based supramolecular nanoparticles for loading and sustained release of ATP. Chin. Chem. Lett. 2018, 29, 989–991. [Google Scholar] [CrossRef]
- Liang, X.; Fan, X.; Li, R.; Li, S.; Shen, S.; Hu, D. Efficient removal of Cr(VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure. Bioresour. Technol. 2018, 250, 178–184. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Q.; Zhao, X.; Wang, Z. Highly Efficient Removal of Copper Ions from Water by Using a Novel Alginate-Polyethyleneimine Hybrid Aerogel. Int. J. Biol. Macromol. 2019, 138, 1079–1086. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Haija, M.A. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Gao, J.; Wang, K.Y.; Chung, T.S. Design of Nanofiltration (NF) Hollow Fiber Membranes Made from Functionalized Bore Fluids Containing Polyethyleneimine (PEI) for Heavy Metal Removal. J. Membr. Sci. 2020, 603, 118022. [Google Scholar] [CrossRef]
- Godiya, C.B.; Sayed, S.M.; Xiao, Y.; Lu, X. Highly Porous Egg White/Polyethyleneimine Hydrogel for Rapid Removal of Heavy Metal Ions and Catalysis in Wastewater. React. Funct. Polym. 2020, 149, 104509. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Wu, W.; Pang, W.; Yan, G. Efficient Removal of Alizarin Red S from Aqueous Solution by Polyethyleneimine Functionalized Magnetic Carbon Nanotubes. Bioresour. Technol. 2019, 293, 122100. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Sun, Z.; Meng, H.; Han, Y.; Wu, J.; Xu, J.; Xu, Y.; Zhang, X. Polyethyleneimine (PEI) Incorporated Cu-BTC Composites: Extended Applications in Ultra-High Efficient Removal of Congo Red. J. Solid State Chem. 2019, 270, 231–241. [Google Scholar] [CrossRef]
- Bektar, M.; Ali Rasekh, H.; Jaafar Soltanianfard, M. Synthesis and Characterization of CoFe2O4@SiO2-Polyethyleneimine Magnetic Nanoparticle and Its Application for Ultrasonic-Assisted Removal of Disulfine Blue Dye from Aqueous Solution. Arab. J. Chem. 2020, 13, 5430–5437. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Ning, J.; Gao, M.; Jiang, W.; Zhou, Z.; Li, G. Preparation and Characterization of a Novel Polyethyleneimine Cation-Modified Persimmon Tannin Bioadsorbent for Anionic Dye Adsorption. J. Environ. Manag. 2018, 217, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Huang, W.; Xu, K.; Li, M.; Fan, M.; Wang, K. Preparation of Anion Exchange Membrane with Branch Polyethyleneimine as Main Skeleton Component. Mater. Des. 2018, 160, 698–707. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, L.; Xu, X.; Wang, H.; Xu, P. Physicochemical and Electrochemical Characterization of Cation-Exchange Membranes Modified with Polyethyleneimine for Elucidating Enhanced Monovalent Permselectivity of Electrodialysis. J. Membr. Sci. 2019, 572, 545–556. [Google Scholar] [CrossRef]
- Pan, J.; Ding, J.; Tan, R.; Chen, G.; Zhao, Y.; Gao, C.; van der Bruggen, B.; Shen, J. Preparation of a Monovalent Selective Anion Exchange Membrane through Constructing a Covalently Crosslinked Interface by Electro-Deposition of Polyethyleneimine. J. Membr. Sci. 2017, 539, 263–272. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Z.; Zhang, F.; Yang, B.; Zhang, S. A novel hydrophilic polymer-based anion exchanger grafted by quaternized polyethyleneimine for ion chromatography. Talanta 2019, 197, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Ohs, B.; Lenhart, J.; Abduly, L.; Blanke, P.; Wessling, M. High capacity polyethylenimine impregnated microtubes made of carbon nanotubes for CO2 capture. Carbon 2018, 126, 338–345. [Google Scholar] [CrossRef]
- Zhang, H.; Goeppert, A.; Kar, S.; Prakash, G.K.S. Structural parameters to consider in selecting silica supports for polyethylenimine based CO2 solid adsorbents. Importance of pore size. J. CO2 Util. 2018, 26, 246–253. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, S.; Rout, K.R.; Chen, D. Development of polyethylenimine (PEI)-impregnated mesoporous carbon spheres for low-concentration CO2 capture. Catal. Today. 2020. [Google Scholar] [CrossRef]
- Jung, W.; Park, J.; Won, W.; Lee, K.S. Simulated moving bed adsorption process based on a polyethylenimine-silica sorbent for CO2 capture with sensible heat recovery. Energy 2018, 150, 950–964. [Google Scholar] [CrossRef]
- Ira, Y.F.; Golenser, J.; Nurit, B.; Weiss, E.; Domb, A. Quaternary Ammonium Polyethyleneimine: Antibacterial Activity. J. Nanomater. 2010, 826343. [Google Scholar] [CrossRef] [Green Version]
- Gibney, K.A.; Sovadinova, I.; Lopez, A.I.; Urban, M.; Ridgway, Z.; Caputo, G.A.; Kuroda, K. Poly(Ethylene Imine)s as Antimicrobial Agents with Selective Activity. Macromol. Biosci. 2012, 12, 1279–1289. [Google Scholar] [CrossRef] [Green Version]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Tuladhar, E.; de Koning, M.C.; Fundeanu, I.; Beumer, R.; Duizer, E. Different Virucidal Activities of Hyperbranched Quaternary Ammonium Coatings on Poliovirus and Influenza Virus. Appl. Environ. Microbiol. 2012, 78, 2456–2458. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency. List N: Disinfectants for Use against SARS-CoV-2. Available online: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2 (accessed on 23 June 2020).
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Uday, S.P.; Thiyagarajan, D.; Goswami, S.; Adhikari, M.D.; Das, G.; Ramesh, A. Amphiphile-mediated enhanced antibiotic efficacy and development of a payload nanocarrier for effective killing of pathogenic bacteria. J. Mater. Chem. B 2014, 2, 5818–5827. [Google Scholar] [CrossRef]
- Gottenbos, B.; Grijpma, D.W.; van der Mei, H.C.; Feijen, J.; Busscher, H.J. Antimicrobial Effects of Positively Charged Surfaces on Adhering Gram-Positive and Gram-Negative Bacteria. J. Antimicrob. Chemother. 2001, 48, 7–13. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall Teichoic Acid Function, Biosynthesis, and Inhibition. Chembiochem 2010, 11, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the Essentiality of Lipopolysaccharide to Gram-Negative Bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, T. Antibacterial and Bacterium Adsorbing Macromolecules. Macromol. Mater. Eng. 2001, 286, 63–87. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, S.; Zhou, X.; Wang, H.; Xu, H.H.K.; Cheng, L. The Use of Quaternary Ammonium to Combat Dental Caries. Materials 2015, 8, 3532–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, A.; Mishra, A. Antibacterial Polymers—A Mini Review. Mater. Today Proc. 2018, 5, 17156–17161. [Google Scholar] [CrossRef]
- Iftekhar, H. Nanocomposite restorative materials for dental caries management. In Applications of Nanocomposite Materials in Dentistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 161–169. [Google Scholar] [CrossRef]
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent Advances in Antimicrobial Polymers: A Mini-Review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [Green Version]
- Ortega, A.; Farah, S.; Tranque, P.; Ocaña, A.V.; Nam-Cha, S.H.; Beyth, N.; Gómez-Roldán, C.; Pérez-Tanoira, R.; Domb, A.J.; Pérez-Martínez, F.C.; et al. Antimicrobial Evaluation of Quaternary Ammonium Polyethyleneimine Nanoparticles against Clinical Isolates of Pathogenic Bacteria. IET Nanobiotechnol. 2015, 9, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles Used in Dentistry: A Review. J. Oral. Biol. Craniofac. Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [Green Version]
- AlKahtani, R.N. The Implications and Applications of Nanotechnology in Dentistry: A Review. Saudi Dent. J. 2018, 30, 107–116. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial Activity of Dental Composites Containing Quaternary Ammonium Polyethylenimine Nanoparticles against Streptococcus Mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Basu, A.; Weiss, E.I.; Domb, A.J. Antimicrobial Nanoparticles in Restorative Composites. In Emerging Nanotechnologies in Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Domb, A.J.; Weiss, E.; Beyth, N.; Farber, I.; Perez-Davidi, M. Antimicrobial Nanoparticulate Additives Forming Non-Leachable Sustained Antimicrobial Polymeric Compositions. U.S. Patent 20080226728A1, 18 September 2008. [Google Scholar]
- Farah, S.; Khan, W.; Ira, Y.F.; Kesler Shvero, D.; Beyth, N.; Weiss, E.; Domb, A. Crosslinked QA-PEI Nanoparticles: Synthesis Reproducibility, Chemical Modifications, and Stability Study. Polym. Advan. Technol. 2013, 24, 446–452. [Google Scholar] [CrossRef]
- Nuzhdina, A.V.; Morozov, A.S.; Kopitsyna, M.N.; Strukova, E.N.; Shlykova, D.S.; Bessonov, I.V.; Lobakova, E.S. Simple and Versatile Method for Creation of Non-Leaching Antimicrobial Surfaces Based on Cross-Linked Alkylated Polyethyleneimine Derivatives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 788–795. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Weiss, E.I.; Domb, A.J. Antibacterial Effect of Composite Resins Containing Quaternary Ammonium Polyethyleneimine Nanoparticles. J. Nanopart. Res. 2010, 12, 591–603. [Google Scholar] [CrossRef]
- Yew, P.Y.M.; Chee, P.L.; Cally, O.; Zhang, K.; Liow, S.S.; Lohn, X.J. Quarternized Short Polyethylenimine Shows Good Activity against Drug-Resistant Bacteria. Macromol. Mater. Eng. 2017, 302, 1700186. [Google Scholar] [CrossRef]
- Azzam, T.; Eliyahu, H.; Shapira, L.; Linial, M.; Barenholz, Y.; Domb, A.J. Polysaccharide-oligoamine based conjugates for gene delivery. J. Med. Chem. 2002, 45, 1817–1824. [Google Scholar] [CrossRef]
- Zaltsman, N.; Kesler-Shvero, D.; Weiss, E.I.; Beyth, N. Synthesis Variants of Quaternary Ammonium Polyethyleneimine Nanoparticles and Their Antibacterial Efficacy in Dental Materials. J. Appl. Biomater. Funct. Mater. 2016, 14, e205–e211. [Google Scholar] [CrossRef] [Green Version]
- Youdovin-Farber, I.; Beyth, N.; Nyska, A.; Weiss, E.I.; Golenser, J.; Domb, A.J. Surface Characterization and Biocompatibility of Restorative Resin Containing Nanoparticles. Biomacromolecules 2008, 9, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Baraness-Hadar, L.; Yudovin-Farber, I.; Domb, A.J.; Weiss, E.I. Surface Antimicrobial Activity and Biocompatibility of Incorporated Polyethylenimine Nanoparticles. Biomaterials 2008, 29, 4157–4163. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Perez-Davidi, M.; Domb, A.J.; Weiss, E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 22038–22043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrokovski, Y.; Nisimov, I.; Kesler-Shvero, D.; Zaltsman, N.; Beyth, N. Antibacterial Effect of Composite Resin Foundation Material Incorporating Quaternary Ammonium Polyethyleneimine Nanoparticles. J. Prosthet. Dent. 2016, 116, 603–609. [Google Scholar] [CrossRef]
- Shvero, D.K.; Zatlsman, N.; Hazan, R.; Weiss, E.I.; Beyth, N. Characterization of the Antibacterial Effect of Polyethyleneimine Nanoparticles in Relation to Particle Distribution in Resin Composite. J. Dent. 2015, 43, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Zaltsman, N.; Kesler Shvero, D.; Polak, D.; Weiss, E.I.; Beyth, N. Antibacterial Orthodontic Adhesive Incorporating Polyethyleneimine Nanoparticles. Oral Health Prev. Dent. 2017, 15, 245–250. [Google Scholar] [CrossRef]
- Varon-Shahar, E.; Sharon, E.; Zabrovsky, A.; Houri-Haddad, Y.; Beyth, N. Antibacterial Orthodontic Cements and Adhesives: A Possible Solution to Streptococcus Mutans Outgrowth Adjacent to Orthodontic Appliances. Oral. Health Prev. Dent. 2019, 17, 49–56. [Google Scholar] [CrossRef]
- Beyth, S.; Polak, D.; Milgrom, C.; Weiss, E.I.; Matanis, S.; Beyth, N. Antibacterial Activity of Bone Cement Containing Quaternary Ammonium Polyethyleneimine Nanoparticles. J. Antimicrob. Chemother. 2014, 69, 854–855. [Google Scholar] [CrossRef] [Green Version]
- Shvero, D.K.; Davidi, M.P.; Weiss, E.I.; Srerer, N.; Beyth, N. Antibacterial Effect of Polyethyleneimine Nanoparticles Incorporated in Provisional Cements against Streptococcus Mutans. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 367–371. [Google Scholar] [CrossRef]
- Abramovitz, I.; Beyth, N.; Weinberg, G.; Borenstein, A.; Polak, D.; Kesler-Shvero, D.; Houri-Haddad, Y. In Vitro Biocompatibility of Endodontic Sealers Incorporating Antibacterial Nanoparticles. J. Nanomater. 2012, 858073. [Google Scholar] [CrossRef] [Green Version]
- Lambert, P.A. Mechanisms of Antibiotic Resistance in Pseudomonas Aeruginosa. J. R. Soc. Med. 2002, 95, 22–26. [Google Scholar]
- Farah, S.; Aviv, O.; Laout, N.; Ratner, S.; Beyth, N.; Domb, A.J. Quaternary Ammonium Polyethylenimine Nanoparticles for Treating Bacterial Contaminated Water. Colloids. Surf. B 2015, 128, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.J.; Snow, G.A. Attack and Defense: Drug Transport across Cell Walls and Membranes. In Biochemistry and Molecular Biology of Antimicrobial Drug Action; Springer: Boston, MA, USA, 2005; pp. 121–134. [Google Scholar]
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef] [Green Version]
- Morrison, R.T.; Boyd, R.N. Chemia Organiczna, 2nd ed.; PWN: Warsaw, Poland, 1990; pp. 533–534. [Google Scholar]
- Hamlin, T.A.; Swart, M.; Bickelhaupt, F.M. Nucleophilic Substitution (SN2): Dependence on Nucleophile, Leaving Group, Central Atom, Substituents, and Solvent. ChemPhysChem 2018, 19, 1315–1330. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [Green Version]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Venkatesh, M.; Barathi, V.A.; Goh, E.T.L.; Anggara, R.; Fazil, M.H.U.T.; Ng, A.J.Y.; Harini, S.; Aung, T.T.; Fox, S.J.; Liu, S.; et al. Antimicrobial activity and cell selectivity of synthetic and biosynthetic cationic polymers. Antimicrob. Agents Chemother. 2017, 61, e00469-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus Mutans, Caries and Simulation Models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Loesche, W.J. Role of Streptococcus Mutans in Human Dental Decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef]
- 3M FiltekTM Z250 Universal Restorative System, Technical Product Profile. Available online: https://multimedia.3m.com/mws/media/78343O/3m-filtek-z250-universal-restorative-technical-product-profile.pdf (accessed on 29 August 2020).
- 3MTM Single Bond Universal Adhesive, Technical Product Profile. Available online: https://multimedia.3m.com/mws/media/1279637O/3m-single-bond-universal-adhesive-technical-product-profile.pdf (accessed on 29 August 2020).
- FiltekTM Supreme XT Flowable Restorative, Technical Product Profile. Available online: https://multimedia.3m.com/mws/media/598213O/filtek-supreme-xt-flow-tpp.pdf (accessed on 29 August 2020).
- FiltekTM Supreme XTE Flowable Restorative, Technical Product Profile. Available online: https://multimedia.3m.com/mws/media/715606O/filtek-supreme-xte-flowable-technical-profile-anz.pdf?fn=supr_xte_fl_tpp.pdf (accessed on 29 August 2020).
- Jefferies, R.S. Abrasive Finishing and Polishing in Restorative Dentistry: A State-of-the-Art Review. Dent. Clin. N. Am. 2007, 51, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Melo, M.A.S.; Chen, C.; Liu, J.; Weir, M.D.; Bai, Y.; Xu, H.K.H. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Perdigāo, J. Current perspectives on dental adhesion: (1) Dentin Adhesion—Not there yet. Jpn. Dent. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Dressano, D.; Salvadro, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef]
- Sharon, E.; Shrabi, R.; Eden, A.; Zabrovsky, A.; Ben-Gal, G.; Sharon, E.; Pietrokovski, Y.; Houri-Haddad, Y.; Beyth, N. Antibacterial Activity of Orthodontic Cement Containing Quaternary Ammonium Polyethylenimine Nanoparticles Adjacent to Orthodontic Brackets. Int. J. Environ. Res. Public Health 2018, 15, 606. [Google Scholar] [CrossRef] [Green Version]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An Essential Material in Medicine and Dentistry. J. Long. Term. Eff. Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, D.A.; Paula, A.B.; Marto, C.M.; Coelho, A.; Paulo, S.; Martinho, J.P.; Carrilho, E.; Ferreira, M.M. Biocompatibility of Root Canal Sealers: A Systematic Review of In Vitro and In Vivo Studies. Materials 2019, 12, 4113. [Google Scholar] [CrossRef] [Green Version]












| Alkyl Bromide. | Crosslinking Agent | Antibacterial Activity 1 | |
|---|---|---|---|
| S. aureus | P. aeruginosa | ||
| 1-bromohexadecane | Ethylene glycol bis(chloroacetate) | 3.2 | <1.5 |
| Diethylene glycol bis(chloroacetate) | 3.4 | <1.5 | |
| Triethylene glycol bis(chloroacetate) | 3.6 | <1.6 | |
| Polyethylene glycol bis(chloroacetate) | 3.8 | <1.7 | |
| 1,5-Dibromopentane | 2.1 | <1.5 | |
| Glutaraldehyde | 2.4 | <1.5 | |
| Alkyl Bromide | Crosslinking Agent | Crosslinking Ratio | Antibacterial Activity 1 | |
|---|---|---|---|---|
| S. aureus | P. aeruginosa | |||
| 1-bromooctane | Ethylene glycol bis(chloroacetate) | 1:0.02 | >6 | 5.0 |
| 1:0.04 | >6 | 4.3 | ||
| 1:0.06 | >6 | 3.8 | ||
| 1:0.08 | 4.8 | 2.5 | ||
| 1:0.1 | 4.6 | <1.8 | ||
| 1-bromododecane | Ethylene glycol bis(chloroacetate) | 1:0.02 | 4.9 | 1.6 |
| 1:0.04 | 4.4 | <1.5 | ||
| 1:0.06 | 4.2 | <1.5 | ||
| 1:0.08 | 3.7 | <1.5 | ||
| 1:0.1 | 3.3 | <1.5 | ||
| 1-bromohexadecane | Ethylene glycol bis(chloroacetate) | 1:0.02 | 3.5 | <1.5 |
| 1:0.04 | 3.2 | <1.5 | ||
| 1:0.06 | 2.8 | <1.5 | ||
| 1:0.08 | 2.6 | <1.5 | ||
| 1:0.1 | 1.8 | <1.5 | ||
| Dental Composite Material/Manufacturer | Chemical Composition | Content of QA-PEI Nanoparticles (wt.%) | Tested Bacteria | Ref. |
|---|---|---|---|---|
| FiltekTM Z250/3MTM | Zirconia/silica filler, Bis-GMA, UDMA, Bis-EMA [112] | 1 | S. mutans | [80] |
| Filtek Flow/3MTM | Zirconia/silica filler, Bis-GMA, TEGDMA | 1 | S. mutans | [80,85] |
| 1 2 | S. aureus, S. epidermis, E. faecalis, P. aeruginosa, E. coli | [90] | ||
| 1 | in vivo studies | [91] | ||
| Single Bond/3MTM | Dimethacrylate resins, HEMA [113] | 1 | S. mutans | [80] |
| Q Core/BJM Laboratories Ltd. | Bis-GMA, TEGDMA, aluminoborosilicate filler, fluoride-releasing filler | 1 | A. viscosus, S. mutans | [92] |
| Filtek Supreme XT Flowable Restorative/3MTM | Zirconia/silica filler Bis-GMA, TEGDMA, Bis-EMA [114] | 0.5 1 2 | E. faecalis | [83,88] |
| Filtek Supreme XTE Flowable Restorative/3MTM | Zirconia/silica filler, fluoride filler, Bis-GMA, TEGDMA, procrylat resins [115] | 1 2 | E. faecalis, S. mutans, A. viscosus, L. casei | [93] |
| Dental Composite Material/Manufacturer | Type of Dental Material | Content of QA-PEI Nanoparticles (wt.%) | Tested Bacteria | Ref. |
|---|---|---|---|---|
| AH Plus/Dentsply DeTrey | Root canal sealer pastes | 0.5 1 2 | E. faecalis | [88] |
| AH 26/Dentsply DeTrey | Root canal sealer pastes | 0.5 1 2 | E. faecalis | [88] |
| BJM RCS/B.J.M. Laboratories | Root canal sealer pastes | 0.5 1 2 | E. faecalis | [88] |
| Single Bond adhesive/3MTM | Bonding resin | 1 | S. mutans | [80] |
| RelyX Temp NE/3M ESPE | Provisional cement | 0.5 1 2 | S. mutans E. faecalis | [97] |
| NeoBond | Orthodontic cement | 1 1.5 | S. mutans | [94] |
| 1 | S. mutans L. casei | [117] | ||
| 1 | S. mutans | [95] | ||
| Transbond Plus/3M Oral Care | Orthodontic adhesive | 1 | S. mutans | [95] |
| Transbond CT/3M Oral Care | Orthodontic adhesive | 1 | S. mutans | [95] |
| GC Fuji ORTHO LC/GC | Orthodontic cement | 1 | S. mutans | [95] |
| GC CX-Plus/SHOFU | Orthodontic cement | 1 | S. mutans | [95] |
| Simplex TM P Bone Cement/Stryker MedEd | Bone cement | 1 2 3 | S. aureus E. faecalis | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrószcz, M.; Barszczewska-Rybarek, I. Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials. Polymers 2020, 12, 2551. https://doi.org/10.3390/polym12112551
Chrószcz M, Barszczewska-Rybarek I. Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials. Polymers. 2020; 12(11):2551. https://doi.org/10.3390/polym12112551
Chicago/Turabian StyleChrószcz, Marta, and Izabela Barszczewska-Rybarek. 2020. "Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials" Polymers 12, no. 11: 2551. https://doi.org/10.3390/polym12112551
APA StyleChrószcz, M., & Barszczewska-Rybarek, I. (2020). Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials. Polymers, 12(11), 2551. https://doi.org/10.3390/polym12112551