Tailoring Multi-Level Structural and Practical Features of Gelatin Films by Varying Konjac Glucomannan Content and Drying Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of KGM/Gelatin Composite Films
2.3. Scanning Electron Microscopy (SEM)
2.4. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Small Angle X-Ray Scattering (SAXS)
2.6. X-Ray Diffraction (XRD)
2.7. Mechanical Properties
2.8. Contact Angle Analysis
2.9. Statistical Analysis
3. Results
3.1. Microscopic Morphology of Fracture Surface
3.2. ATR-FTIR Spectroscopy
3.3. Nano-Structural Characteristics
3.4. Crystalline Structural Characteristics
3.5. Mechanical Features
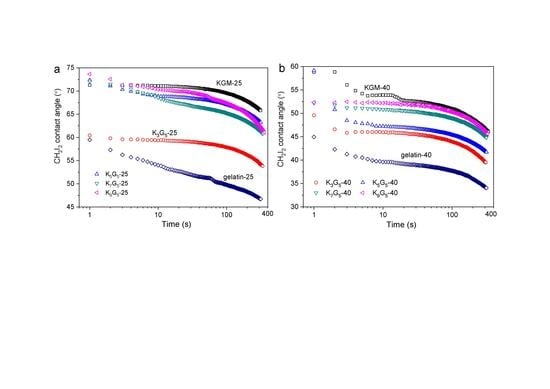
3.6. CH2I2 Contact Angle Features
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.J.; Haugaard, V.; Festersen, R.; Bertelsen, G. Production and applications of biobased packaging materials for the food industry. Food Addit. Contam. 2002, 19, 172–177. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Rouhi, M.; Kariminejad, M.; Mortazavian, A.M.; Sadeghi, E.; Hasanvand, S. Physico-mechanical and structural properties of eggshell membrane gelatin-chitosan blend edible films. Int. J. Biol. Macromol. 2018, 107, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Li, X.; Jiang, F.; Ni, X.; Yan, W.; Fang, Y.; Corke, H.; Xiao, M. Preparation and characterization of konjac glucomannan and ethyl cellulose blend films. Food Hydrocoll. 2015, 44, 229–236. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Chen, Y.; Chen, Y.; Chang, P.R. Structural characterization and properties of starch/konjac glucomannan blend films. Carbohydr. Polym. 2008, 74, 946–952. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Li, B.; Kennedy, J.F.; Jiang, Q.G.; Xie, B.J. Quick dissolvable, edible and heatsealable blend films based on konjac glucomannan—Gelatin. Food Res. Int. 2006, 39, 544–549. [Google Scholar] [CrossRef]
- Ye, X.; Kennedy, J.F.; Li, B.; Xie, B.J. Condensed state structure and biocompatibility of the konjac glucomannan/chitosan blend films. Carbohydr. Polym. 2006, 64, 532–538. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Galactomannans use in the development of edible films/coatings for food applications. Trends Food Sci. Technol. 2011, 22, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, N.M.; Paiva, B.; Camassola, M.; Rosenthal-Kim, E.Q.; Garcia, K.C.; dos Santos, F.P.; Soares, R.M.D. Gelatin and galactomannan-based scaffolds: Characterization and potential for tissue engineering applications. Carbohydr. Polym. 2015, 133, 8–18. [Google Scholar] [CrossRef]
- Katsuraya, K.; Okuyama, K.; Hatanaka, K.; Oshima, R.; Sato, T.; Matsuzaki, K. Constitution of konjac glucomannan: Chemical analysis and 13C NMR spectroscopy. Carbohydr. Polym. 2003, 53, 183–189. [Google Scholar] [CrossRef]
- Nishinari, K. Konjac Glucomannan. Dev. Food Sci. 2000, 41, 309–330. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, Q.; Qian, H.; Xiao, M.; Corke, H.; Nishinari, K.; Jiang, F.T. Controllable hydrophilicity-hydrophobicity and related properties of konjac glucomannan and ethyl cellulose composite films. Food Hydrocoll. 2018, 79, 301–309. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, Y.; Gao, S.; Zhang, L. Characterization of konjac glucomannan–gelatin blend films. J. Appl. Polym. Sci. 2001, 79, 1596–1602. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Zhang, K.; Li, J.; Hou, H. Novel hard capsule prepared by tilapia (Oreochromis niloticus) scale gelatin and konjac glucomannan: Characterization, and in vitro dissolution. Carbohydr. Polym. 2019, 206, 254–261. [Google Scholar] [CrossRef]
- Tomczynska-Mleko, M.; Brenner, T.; Nishinari, K.; Mleko, S.; Kramek, A. Rheological and thermal behavior of mixed gelatin/konjac glucomannan gels. J. Texture Stud. 2014, 45, 344–353. [Google Scholar] [CrossRef]
- Jin, W.; Xu, W.; Ge, H.; Li, J.; Li, B. Coupling process of phase separation and gelation in konjac glucomannan and gelatin system. Food Hydrocoll. 2015, 51, 188–192. [Google Scholar] [CrossRef]
- Qiao, D.; Tu, W.; Zhang, B.; Wang, R.; Li, N.; Nishinari, K.; Riffat, S.; Jiang, F. Understanding the multi-scale structure and digestion rate of water chestnut starch. Food Hydrocoll. 2019, 91, 311–318. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, W.; Qiao, D.; Zhang, P.; Zhao, S.; Zhang, L.; Xie, F. Changes in Nanoscale Chain Assembly in Sweet Potato Starch Lamellae by Downregulation of Biosynthesis Enzymes. J. Agric. Food. Chem. 2019, 67, 6302–6312. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Cai, Z.; Guo, Y.; Xu, T.; Qiao, D.; Zhang, B.; Zhao, S.; Huang, Q.; Niu, M.; Jia, C.; et al. Hierarchical structure and slowly digestible features of rice starch following microwave cooking with storage. Food Chem. 2019, 295, 475–483. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; He, K.; Wang, N. The Effects of Different Plasticizers on the Properties of Thermoplastic Starch as Solid Polymer Electrolytes. Macromol. Mater. Eng. 2007, 292, 503–510. [Google Scholar] [CrossRef]
- Zhang, B.; Gilbert, E.P.; Qiao, D.; Xie, F.; Wang, D.K.; Zhao, S.; Jiang, F. A further study on supramolecular structure changes of waxy maize starch subjected to alkaline treatment by extended-q small-angle neutron scattering. Food Hydrocoll. 2019, 95, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Qiao, D.; Xie, F.; Zhang, B.; Zou, W.; Zhao, S.; Niu, M.; Lv, R.; Cheng, Q.; Jiang, F.; Zhu, J. A further understanding of the multi-scale supramolecular structure and digestion rate of waxy starch. Food Hydrocoll. 2017, 65, 24–34. [Google Scholar] [CrossRef]
- Peña, C.; de la Caba, K.; Eceiza, A.; Ruseckaite, R.; Mondragon, I. Enhancing water repellence and mechanical properties of gelatin films by tannin addition. Bioresour. Technol. 2010, 101, 6836–6842. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Liu, J.; Xie, F.; Chen, L. Supramolecular structure of A- and B-type granules of wheat starch. Food Hydrocoll. 2013, 31, 68–73. [Google Scholar] [CrossRef]
- Li, N.; Niu, M.; Zhang, B.; Zhao, S.; Xiong, S.; Xie, F. Effects of concurrent ball milling and octenyl succinylation on structure and physicochemical properties of starch. Carbohydr. Polym. 2017, 155, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Majeed, H.; Antoniou, J.; Li, Y.; Ma, Y.; Yokoyama, W.; Ma, J.; Zhong, F. Tailoring physical properties of transglutaminase-modified gelatin films by varying drying temperature. Food Hydrocoll. 2016, 58, 20–28. [Google Scholar] [CrossRef]
- Marszalek, P.E.; Oberhauser, A.F.; Pang, Y.-P.; Fernandez, J.M. Polysaccharide elasticity governed by chair–boat transitions of the glucopyranose ring. Nature 1998, 396, 661–664. [Google Scholar] [CrossRef]
- Qiao, D.; Li, S.; Yu, L.; Zhang, B.; Simon, G.; Jiang, F. Effect of alkanol surface grafting on the hydrophobicity of starch-based films. Int. J. Biol. Macromol. 2018, 112, 761–766. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, D.; Wang, Z.; Cai, C.; Yin, S.; Qian, H.; Zhang, B.; Jiang, F.; Fei, X. Tailoring Multi-Level Structural and Practical Features of Gelatin Films by Varying Konjac Glucomannan Content and Drying Temperature. Polymers 2020, 12, 385. https://doi.org/10.3390/polym12020385
Qiao D, Wang Z, Cai C, Yin S, Qian H, Zhang B, Jiang F, Fei X. Tailoring Multi-Level Structural and Practical Features of Gelatin Films by Varying Konjac Glucomannan Content and Drying Temperature. Polymers. 2020; 12(2):385. https://doi.org/10.3390/polym12020385
Chicago/Turabian StyleQiao, Dongling, Zhong Wang, Chi Cai, Song Yin, Hong Qian, Binjia Zhang, Fatang Jiang, and Xiang Fei. 2020. "Tailoring Multi-Level Structural and Practical Features of Gelatin Films by Varying Konjac Glucomannan Content and Drying Temperature" Polymers 12, no. 2: 385. https://doi.org/10.3390/polym12020385
APA StyleQiao, D., Wang, Z., Cai, C., Yin, S., Qian, H., Zhang, B., Jiang, F., & Fei, X. (2020). Tailoring Multi-Level Structural and Practical Features of Gelatin Films by Varying Konjac Glucomannan Content and Drying Temperature. Polymers, 12(2), 385. https://doi.org/10.3390/polym12020385