1. Introduction
The rapid development of the highway has received much attention in the past few years because of its perfect pavement properties, operational safety and convenience [
1,
2,
3]. Asphalt mixtures (or concrete) have been utilized in more than 90% of completed highway constructions and homogeneous buildings. However, pavement distress happens frequently with increasing use of asphalt pavements [
4,
5] and can even be observed during operation times [
6,
7,
8]. The moisture damage to asphalt pavement is a major problem that negatively affects pavement performance, due to its early appearance, the rapid speed with which damage to the pavement occurs, the enormous effect on comfort and safety during driving, and so on [
9,
10,
11,
12]. That is to say, moisture damage can significantly shorten the repair cycles and the lifetime of asphalt highway, accordingly increasing the cost of maintenance and repair [
13,
14]. Therefore, it is necessary to study the interfacial adhesive properties between the acidic aggregate and the asphalt [
15], due to the important economic and social significances [
16,
17].
The most important reason for the occurrence of moisture damage is the poor interfacial adhesive property between the asphalt and the aggregate [
18]. Currently, an increasing number of technologies for enhancing the interfacial bonding property are being studied, such as giving priority to alkaline aggregates, or employing anti-stripping agents (ASA) [
19,
20]. However, these have not been implemented, for reasons as follows. First of all, alkaline aggregates are better able to bond to asphalt than acidic ones, but some of them (e.g., limestone) are not as rigid and compactible as the acidic ones, resulting in fundamental limitations in the pavement performance of asphalt mixtures. Additionally, alkaline aggregates (e.g., basalt) are suffering from resource depletion in many areas of the world, leading to other unsatisfactory substitute mineral aggregates being employed, rather than the desired ones, which would require great transportation expenses [
21,
22]. Furthermore, ASA may help to improve the adhesive properties between neutral minerals and asphalt, but they are always insufficient for acidic ones, showing unsatisfactory effects on different kinds of acidic aggregates. Additionally, current anti-stripping products may lead to thermal decomposition at high temperatures in asphalt mixing plants and transportation trucks [
23,
24]. Therefore, they are not able to improve the interfacial bonding strength between acidic aggregates and asphalt after mixing and construction. Once the water enters the asphalt concrete, hydrogen bonding can take place on the hydrophilic surface of the aggregates, displacing asphalt from the aggregate and causing stripping of the asphalt membrane [
16,
25]. In actual fact, mineral aggregates are often partly composed of a mixture of neutral and acidic aggregates, and it is necessary to develop new technologies or ASA materials in order to enhance the interfacial adhesive property between acidic aggregates and asphalt.
Gao et al. [
26] studied four kinds of mineral with reference to the different interfacial models between aggregates and asphalt, where the bonding strength in the neutral mineral model was determined using Van der Waals’ force, while the alkaline mineral model was determined based on electrostatic attraction. Nevertheless, acidic aggregates have not been used, although they may employ a different model. Xu et al. [
27] also modeled the interface between silica and asphalt, where interfacial failure was primarily attributed to bonding failure, based on the tensile simulation carried out. Meanwhile, Yao et al. [
28] focused on the bonding performances between aggregates and aging asphalts, where the aging group of carboxylic acid was able to reinforce the bonding energy. Li et al. [
29] investigated the bonding properties at different temperatures and with different oxide types at the interfaces between the aggregates and asphaltene. Chen et al. [
1] studied the interfacial adhesion mechanism using surface microtopography, and compared different aggregates of limestone and granite, revealing that the degree of surficial texture complexity of limestone and granite decreased after abrasion, the relative flat site increased, and the surficial texture of limestone had a greater degree of influence on the interface. Chu et al. [
11] employed molecular dynamics to simulate the interfacial adhesive properties between aggregates (a-quartz and calcite) and asphalts, suggesting that the surface anisotropy of the aggregates had a significant impact on the interface. Furthermore, Wang et al. [
30] simulated two kinds of models, of which one was the interface between quartz and asphalt, and the other was with quartz, water and asphalt. Predictably, water decreased the bonding strength dramatically. Additionally, Fischer et al. [
31] studied the interfacial adhesive strengths between aggregates and asphalt through atomic force microscopy (AFM), where the surface roughness had a stronger influence compared with the chemical nature of the surface.
As a result, the interfacial adhesion properties between acidic aggregates and asphalt have not yet been thoroughly reported, and the anti-stripping products that are available on the market always exhibit weaker thermal stability, leading to unsatisfactory construction. Therefore, in this study, in order to improve thermal stability and for its effective application between acidic aggregates and asphalt, a novel anti-stripping composite was prepared, and the thermal, adhesive, and pavement performances are discussed in comparison with other ASA products.
2. Experiment
2.1. Materials
To study the interface adhesive performance between asphalt and aggregate, two brands of matrix asphalts KL-90# and SK-90# were employed, and their physical properties are shown in
Table 1 and
Table 2. Two other anti-stripping products were used for comparison, and are referred to as ASA-1 and ASA-2, and it is possible to purchase ASA-1 and ASA-2 on the market in different countries. Meanwhile, three kinds of acidic aggregates were chosen: granite, sandstone and quartzite. Obviously, alkaline aggregates are not considered as a sample in this work due to their better adhesive properties with asphalt. The information regarding the acidic aggregates are shown in
Table 3,
Table 4 and
Table 5.
2.2. Preparation of OMMT/PAR and MAS
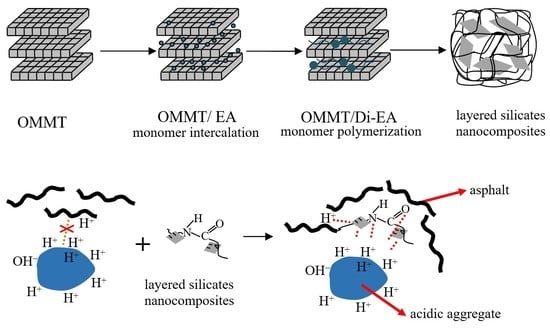
Anti-stripping composites were prepared according to
Figure 1, where eleostearic acid (EA) and organic montmorillonoid (OMMT) were mechanically stirred under CO
2 and gradually heated to 280 °C for 4 h, with OMMT/eleostearic dimer acid nanocomposites (OMMT/Di-EA) being obtained. Then the OMMT/Di-EA were gradually added into the diethylenetriamine (EDTA) system, which was heated to 120 °C under protection by N
2; the system was kept under 200 °C for 2 h before EDTA was distilled under vacuum, and the resulting liquid was a polyamide nanocomposite solution. Moreover, diluent and compatilizer were added under mechanical stirring to achieve a uniform mixture when the temperature was reduced to 80 °C, and the anti-stripping layered silicate nanocomposites of OMMT/Polyamide (OMMT/PAR) were finally achieved. For the purpose of easily injecting it into the mix plant, OMMT/PAR was also diluted by glycerol in a ratio of 5:1 (OMMT/PAR: glycerol), referred to as modification anti-stripping material (MAS). PAR with no OMMT was also prepared in this work.
2.3. Preparation of Asphalt Mixtures
Matrix asphalts were modified by 0.3 wt % OMMT/PAR, ASA-1, and ASA-2, respectively, where the KL-90# modified by OMMT/PAR, ASA-1 and ASA-2 was named as KL-90-OMMT/PAR, KL-90-ASA-1, and KL-90-ASA-1, respectively. Similarly, the names SK-90-OMMT/PAR, SK-90-ASA-1 and SK-90-ASA-2 were used when the asphalt was replaced by SK-90#. Matrix asphalt should always be assumed to be KL-90 unless otherwise stated.
Asphalt concrete AC 16 with a maximum grain size of 16 mm was prepared as the asphalt mixture in this work due to the particle reinforced composites and the common application on highly trafficked road pavements according to the Technical Specification for Construction of Highway Asphalt Pavements (JTG F40, TSCHAP). First of all, the modified asphalts and the acidic aggregates were heated to 150 °C and 160 °C, respectively. The hot modified asphalts and aggregates were mixed at 150 °C for 60 s, and then impacted for the revolving compaction sample with dimensions of 100 ± 2 mm in diameter and 150 ± 2.5 mm in height, and for the Marshall sample, with dimensions of 101.6 ± 0.25 mm in diameter and 63.5 ± 1.3 mm in height, according to the Standard Test Methods of Bitumen and Bituminous Mixture for Highway Engineering (JTG E20, STMBM).
2.4. Thermogravimetry (TG) Analysis
By using the TG-DTA thermal analyzer (Shimadzu Corp., Model DTG-60, Tokyo, Japan), the thermal performances were characterized. The test conditions were as follows: 10 mg samples were placed in an aluminum container and tested from room temperature to 800 °C at a rate of 10 °C/min.
2.5. Adhesive Property Test
According to the industrial standard STMBM, the adhesion properties between asphalts and aggregates are tested by utilizing the poaching method according to the rule (T0616) of STMBM. The aggregates with sizes bigger than 13.2 mm were washed and immersed into a hot asphalt sample after being heated to 105 °C and immersed for 45 s, and were then lifted out. The aggregates with the asphalt were boiled in water for 3 min after cooling in room temperature for 15 min, and the adhesion level can be marked up as having a grade of 1–5 according to the movement of hydration on the aggregate rock surface and the desquamation of the asphalt, for which grade 1 is the lowest grade and grade 5 is the highest one. To confirm the durability, adhesive tests between acidic aggregates and the modified asphalts were also carried out after aging.
2.6. Freeze–Thaw Splitting and Dynamic Elasticity Modulus Test of Bituminous Mixture
The freeze–thaw splitting tests divided the Marshall samples into two groups: A and B. Firstly, the A group samples were immersed in water for 15 min in a vacuum and 30 min under normal pressure; then, they were frozen at −18 °C for 16 h, and immersed in 60 °C water for 24 h. Finally, the A and B group samples were all immersed in 25 °C water for 2 h and the splitting strengths were tested, respectively. The splitting strengths of the B group were defined as the splitting strengths of these samples. Moreover, the ratio of splitting strengths of the A and B groups were defined as the splitting strength ratios (TSR) of these samples according to STMBM. The dynamic elasticity moduli of the asphalt mixtures were also evaluated according to STMBM under a temperature of 20 °C and a frequency of 10 Hz.
4. Adhesion Mechanism Analysis
The interfacial adhesion mechanism between the asphalt and acidic aggregate is shown in
Figure 7. There are abundant hydrogen ions or groups on the molecular chain due to the specific asphalt manufacturing technique, thus asphalt is generally acidic. When asphalt mixes with an alkaline aggregate, a neutralization reaction occurs between the acidic group of the asphalt and the alkaline group of the aggregate. Furthermore, tight adhesion can be confirmed, and this is why asphalt possesses better adhesion capability with alkaline aggregates than acidic ones. In contrast, when asphalt and acidic aggregates are mixed, repulsion interactions take place between the acidic groups of the asphalt and the aggregate, with only partial interfaces (surfaces without or with a few hydrogen ions) performing adhesion in the mixing procedure, and asphalt envelops the aggregate only as a result of its viscidity on other surfaces. Once the asphalt mixtures have been placed in a moist environment, electronegative hydroxyls in water can easily enter into asphalt/acidic aggregate interfaces due to the attraction of positive charge, which causes moisture damage to the pavement.
For the asphalt mixture using acidic aggregates, their surfaces are wrapped with hydrogen ions from OMMT/PAR; the N and O in OMMT/PAR can combine with the H from the aggregates’ surface by means of a hydrogen bond. Furthermore, OMMT layers in OMMT/PAR have a parallel composition compared to aggregates, are negatively charged, and can be electrostatically attracted by a positive charge on the aggregates’ surface, according to electronic theory, and the surfaces are coated with OMMT/PAR molecules; H atoms present in asphalt can combine with OMMT/PAR through hydrogen bonds. According to the solubility parameter close principle, macromolecular asphalt completely adheres to aggregate coated with abundant macromolecular OMMT/PAR, and the adhesion between the acidic aggregate and the asphalt is dramatically improved, resulting in a better thermal stability at the interfaces.