CD133 Targeted PVP/PMMA Microparticle Incorporating Levamisole for the Treatment of Ovarian Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LEVA/PVP/PMMA Microparticle Preparation
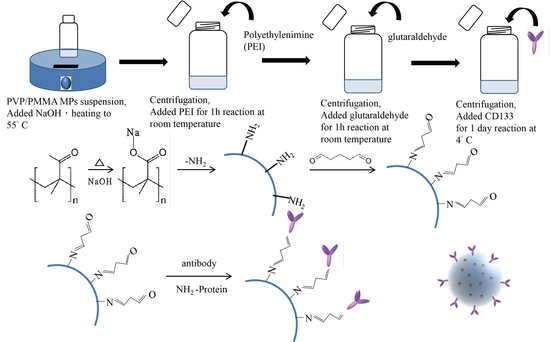
2.3. CD133-Conjugated LEVA/PVP/PMMA MP Preparation
2.4. LEVA/PVP/PMMA MP Surface Morphology and Structural Characterization
2.5. In vitro Release Test
2.6. In Vitro Cell Viability or Cytotoxicity Studies toward SKOV-3 and CP70 Cells
2.7. Surface Morphology and Chemical Composition of Pure LEVA/PVP/PMMA or CD133-Conjugated LEVA/PVP/PMMA Microparticles Using Scanning Electron Microscopy (SEM) and Fourier Transformed-Infrared (FT-IR) Spectroscopy
2.8. Statistical Analyses
3. Results and Discussion
3.1. MP Surface Morphological and Structural Characterization Using Field-Emission SEM and TEM
3.2. In Vitro Release Profile
3.3. Preparation of CD133-Conjugated LEVA/PVP/PMMA Microparticles
3.4. Characterization of CD133-Conjugated LEVA/PVP/PMMA Microparticles
3.5. Cytotoxicity of CD133-Antibody LEVA/PVP/PMMA MPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agarwal, R.; Kaye, S.B. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Siegiel, R.; Miller, K.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.-T.; Chen, Z.-Y.; Sun, C.-Y.; Li, H.-J.; Wang, H.-X.; Cheng, Q.-Q.; Zuo, Z.-Q.; Wang, J.-L.; Liu, Y.-Z.; Wang, Y.-C. Overcoming tumor resistance to cisplatin by cationic lipid-assisted prodrug nanoparticles. Biomaterials 2016, 94, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Kalscheuer, S.M.; Panyam, J. Exploiting nanotechnology to overcome tumor drug resistance: Challenges and opportunities. Adv. Drug Deliv. Rev. 2013, 65, 1731–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livney, Y.D.; Assaraf, Y.G. Rationally designed nanovehicles to overcome cancer chemoresistance. Adv. Drug Deliv. Rev. 2013, 65, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, K.; Zhang, X.-Q.; Pridgen, E.M.; Park, G.Y.; Cui, D.S.; Shi, J.; Wu, J.; Kantoff, P.W.; Lippard, S.J. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc. Natl. Acad. Sci. USA 2013, 110, 18638–18643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Six, J.L.; Ferji, K. Polymerization induced self-assembly: An opportunity toward the self-assembly of polysaccharide-containing copolymers into high-order morphologies. Polymer Chem. 2019, 10, 45–53. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Microparticles Used as Drug Delivery Systems. Smart Colloid. Mater. 2006, 133, 15–21. [Google Scholar]
- Singh, M.N.; Hemant, K.S.Y.; Ram, M.; Shivakumar, H.G. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Cho, R.W.; Clarke, M.F. Recent advances in cancer stem cells. Curr. Opin. Genet. Dev. 2008, 18, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Boman, M.; Wicha, M.S. Cancer stem cells: A step toward the cure. J. Clin. Oncol. 2008, 26, 2795–2799. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.F.; Hass, A.T. Cancer stem cells. In Reviews in Cell Biology and Molecular Medicine; Wiley: Weinheim, Germany, 2006; pp. 221–241. [Google Scholar]
- Bauer, N.; Fonseca, A.-V.; Florek, M.; Freund, D.; Jászai, J.; Bornhäuser, M.; Fargeas, C.A.; Corbeil, D. New insights into the cell biology of hematopoietic progenitors by studying prominin-1 (CD133). Cells Tissues Organs 2008, 188, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulasov, I.V.; Nandi, S.; Dey, M.; Sonabend, A.M.; Lesniak, M.S. Inhibition of Sonic hedgehog and Notch pathways enhances sensitivity of CD133+ glioma stem cells to temozolomide therapy. Mol. Med. 2011, 17, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, M.; Tian, H.; de Sauvage, F.J. The hedgehog signaling pathway in cancer. Clin. Cancer Res. 2006, 12, 5924–5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappa, G.; Fodstad, O.; Lorico, A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem cells 2008, 26, 3008–3017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AMak, B.; Nixon, A.M.; Kittanakom, S.; Stewart, J.M.; Chen, G.I.; Curak, J.; Gingras, A.-C.; Mazitschek, R.; Neel, B.G.; Stagljar, I. Regulation of CD133 by HDAC6 promotes β-catenin signaling to suppress cancer cell differentiation. Cell Rep. 2012, 2, 951–963. [Google Scholar]
- Takenobu, H.; Shimozato, O.; Nakamura, T.; Ochiai, H.; Yamaguchi, Y.; Ohira, M.; Nakagawara, A.; Kamijo, T. CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene 2011, 30, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wu, P.Y. CD133 as a marker for cancer stem cells: Progresses and concerns. Stem Cells Dev. 2009, 18, 1127–1134. [Google Scholar] [CrossRef]
- Taal, B.G.; Tinteren, H.V.; Zoetmulder, F.A.N. Adjuvant 5FU plus levamisole in colonic or rectal cancer: Improved survival in stage II and III. Br. J. Cancer 2001, 85, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Yo, Y.T.; Huang, R.L.; Wang, Y.C.; Liao, Y.P.; Huang, T.S.; Chao, T.K.; Lin, C.K.; Weng, S.J.; Ma, K.H.; et al. Ovarian cancer stem-like cells with induced translineage-differentiation capacity and are suppressed by alkaline phosphatase inhibitor. Oncotarget 2013, 4, 2366–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Bagby, T.R.; Cohen, M.S.; Forrest, M.L. Drug delivery to the lymphatic system: Importance in future cancer diagnosis and therapies. Expert Opin. Drug Deliv. 2009, 6, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Mei, F.; Bai, M.-Y.; Zhao, S.; Chen, D.-R. Release profile characteristics of biodegradable-polymer-coated drug particles fabricated by dual-capillary electrospray. J. Control. Release 2010, 145, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.-Y.; Yang, H.-C. Fabrication of novel niclosamide-suspension using an electrospray system to improve its therapeutic effects in ovarian cancer cells in vitro. Coll. Surf. A Physicochem. Eng. Asp. 2013, 419, 248–256. [Google Scholar] [CrossRef]
- Lin, C.-K.; Bai, M.-Y.; Hu, T.-M.; Wang, Y.-C.; Chao, T.-K.; Weng, S.-J.; Huang, R.-L.; Su, P.-H.; Lai, H.-C. Preclinical evaluation of a nanoformulated antihelminthic, niclosamide, in ovarian cancer. Oncotarget 2016, 7, 8993–9006. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.-L.; Bai, M.-Y.; Wang, J.-Y.; Hong, P.-D. Development and application of micro-polysaccharide drug carriers incorporating doxorubicin and superparamagnetic iron oxide for bimodality treatment of hepatocellular carcinoma. Coll. Surf. B Biointerfaces 2017, 151, 304–313. [Google Scholar] [CrossRef]
- Bai, M.-Y.; Tang, S.-L.; Chuang, M.-H.; Wang, T.-Y.; Hong, P.-D. Evaluation of chitosan derivative microparticles encapsulating superparamagnetic Iron oxide and doxorubicin as a pH-sensitive delivery carrier in hepatic carcinoma treatment: An in vitro comparison study. Front. Pharmacol. 2018, 9, 1025. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.-Y.; Liu, S.-Z. A simple and general method for preparing antibody-PEG-PLGA sub-micron particles using electrospray technique: An in vitro study of targeted delivery of cisplatin to ovarian cancer cells. Coll. Surf. B Biointerfaces 2014, 117, 346–353. [Google Scholar] [CrossRef]
- Silva, I.A.; Bai, S.; McLean, K.; Yang, K.; Griffith, K.; Thomas, D.; Ginestier, C.; Johnston, C.; Kueck, A.; Reynolds, R.K. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011, 71, 3991–4001. [Google Scholar] [CrossRef] [Green Version]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-C.; Bai, M.-Y.; Yeh, Y.-T.; Tang, S.-L.; Yu, M.-H. CD133 Targeted PVP/PMMA Microparticle Incorporating Levamisole for the Treatment of Ovarian Cancer. Polymers 2020, 12, 479. https://doi.org/10.3390/polym12020479
Wang Y-C, Bai M-Y, Yeh Y-T, Tang S-L, Yu M-H. CD133 Targeted PVP/PMMA Microparticle Incorporating Levamisole for the Treatment of Ovarian Cancer. Polymers. 2020; 12(2):479. https://doi.org/10.3390/polym12020479
Chicago/Turabian StyleWang, Yu-Chi, Meng-Yi Bai, Ying-Ting Yeh, Sung-Ling Tang, and Mu-Hsien Yu. 2020. "CD133 Targeted PVP/PMMA Microparticle Incorporating Levamisole for the Treatment of Ovarian Cancer" Polymers 12, no. 2: 479. https://doi.org/10.3390/polym12020479
APA StyleWang, Y.-C., Bai, M.-Y., Yeh, Y.-T., Tang, S.-L., & Yu, M.-H. (2020). CD133 Targeted PVP/PMMA Microparticle Incorporating Levamisole for the Treatment of Ovarian Cancer. Polymers, 12(2), 479. https://doi.org/10.3390/polym12020479