Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Arabinoxylan
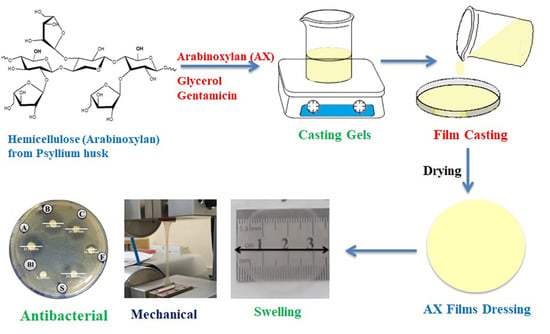
2.3. Preparation of AX Films
2.4. Physical Assessment of Films
2.5. Characterization of Films
2.5.1. Measurement of Thickness
2.5.2. Solvent Loss
2.5.3. Mechanical Characterization of Films
2.5.4. Determination of Extent of Swelling
2.5.5. Water Vapor Transmission Rate (WVTR)
2.5.6. Fourier Transform Infrared Analysis
2.5.7. Thermal Analysis
2.5.8. Scanning Electron Microscopy
2.6. In Vitro Drug Release Study
2.7. Antibacterial Activity
2.8. Cell Viability Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Arabinoxylan
3.2. Preparation and Physical Assessment of AX Films
3.3. Characterization of AX Films
3.3.1. Film Thickness
3.3.2. Solvent Loss
3.3.3. Mechanical Characterization
3.3.4. Swelling Index of the Films
3.3.5. Water Vapor Transmission Rate (WVTR)
3.3.6. Fourier Transform Infrared Analysis
3.3.7. Thermal Analysis
3.3.8. Scanning Electron Microscopy
3.4. Antibiotic Release Study
3.5. Antibacterial Activity
3.6. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pawar, H.V.; Boateng, J.S.; Ayensu, I.; Tetteh, J. Multifunctional medicated lyophilised wafer dressing for effective chronic wound healing. J. Pharm. Sci. 2014, 103, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, S.; Dhiman, A. Design of antibiotic containing hydrogel wound dressings: Biomedical properties and histological study of wound healing. Int. J. Pharm. 2013, 457, 82–91. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and optimization of chitosan-gelatin films for sustained delivery of lupeol for wound healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Huang, K.-Y.; Lew, W.-Z.; Fan, K.-H.; Chang, W.-J.; Huang, H.-M. Gamma-irradiation-prepared low molecular weight hyaluronic acid promotes skin wound healing. Polymers 2019, 11, 1214. [Google Scholar] [CrossRef] [Green Version]
- Kirketerp-Møller, K.; Zulkowski, K.; James, G. Chronic wound colonization, infection, and biofilms. In Biofilm infections; Springer: Berlin/Heidelberg, Germany, 2011; pp. 11–24. [Google Scholar]
- Phan, T.T.V.; Huynh, T.-C.; Oh, J. Photothermal Responsive Porous Membrane for Treatment of Infected Wound. Polymers 2019, 11, 1679. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.-F.; Leow, H.-L. Development of biofilm-targeted antimicrobial wound dressing for the treatment of chronic wound infections. Drug Dev. Ind. Pharm. 2015, 41, 1902–1909. [Google Scholar] [CrossRef]
- Said, J.; Dodoo, C.C.; Walker, M.; Parsons, D.; Stapleton, P.; Beezer, A.E.; Gaisford, S. An in vitro test of the efficacy of silver-containing wound dressings against Staphylococcus aureus and Pseudomonas aeruginosa in simulated wound fluid. Int. J. Pharm. 2014, 462, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Bendy, J.R.; Nuccio, P.; Wolfe, E.; Collins, B.; Tamburro, C.; Glass, W.; Martin, C. Relationship of quantitative wound bacterial counts to healing of decubiti: Effect of topical gentamicin. Antimicrob. Agents Chemother. 1964, 10, 147–155. [Google Scholar]
- Boateng, J.S.; Pawar, H.V.; Tetteh, J. Polyox and carrageenan based composite film dressing containing anti-microbial and anti-inflammatory drugs for effective wound healing. Int. J. Pharm. 2013, 441, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Alavi, T.; Rezvanian, M.; Ahmad, N.; Mohamad, N.; Ng, S.-F. Pluronic-F127 composite film loaded with erythromycin for wound application: Formulation, physicomechanical and in vitro evaluations. Drug Deliv. Transl. Res. 2019, 9, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.; Duerden, B.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, H.; Tetteh, J.; Boateng, J. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf. B 2013, 102, 102–110. [Google Scholar] [CrossRef]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Samadian, H.; Mahakizadeh, S.; Tajerian, R.; Rahmati, M.; Vaez, A.; Salehi, M. Taurine-loaded poly (ε-caprolactone)/gelatin electrospun mat as a potential wound dressing material: In vitro and in vivo evaluation. J. Bioact. Compat. Polym. 2018, 33, 282–294. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Rezvanian, M.; Amin, M.C.I.M.; Ng, S.-F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Jakubowska, E.; Tarach, I.; Sedlarik, V.; Pummerova, M. Antibacterial Films Based on PVA and PVA–Chitosan Modified with Poly (Hexamethylene Guanidine). Polymers 2019, 11, 2093. [Google Scholar] [CrossRef] [Green Version]
- Rezk, A.I.; Lee, J.Y.; Son, B.C.; Park, C.H.; Kim, C.S. Bi-layered Nanofibers Membrane Loaded with Titanium Oxide and Tetracycline as Controlled Drug Delivery System for Wound Dressing Applications. Polymers 2019, 11, 1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.-w.; Ren, J.-l.; Zhong, L.-x.; Sun, R.-c. Nanocomposite films based on xylan-rich hemicelluloses and cellulose nanofibers with enhanced mechanical properties. Biomacromolecules 2011, 12, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.; Iqbal, M.S.; Wolf, B.; Mariam, I.; Rao, S. Comparative drug loading and release study on some carbohydrate polymers. Lat. Am. J. Pharm. 2016, 35, 146–155. [Google Scholar]
- Amin, M.; Iram, F.; Iqbal, M.S.; Saeed, M.Z.; Raza, M.; Alam, S. Arabinoxylan-mediated synthesis of gold and silver nanoparticles having exceptional high stability. Carbohydr. Polym. 2013, 92, 1896–1900. [Google Scholar] [CrossRef] [PubMed]
- Akbar, J.; Iqbal, M.S.; Massey, S.; Masih, R. Kinetics and mechanism of thermal degradation of pentose-and hexose-based carbohydrate polymers. Carbohydr. Polym. 2012, 90, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Akbar, J.; Iqbal, M.S.; Chaudhary, M.T.; Yasin, T.; Massey, S. A QSPR study of drug release from an arabinoxylan using ab initio optimization and neural networks. Carbohydr. Polym. 2012, 88, 1348–1357. [Google Scholar] [CrossRef]
- Bhatia, M.; Ahuja, M. Psyllium arabinoxylan: Carboxymethylation, characterization and evaluation for nanoparticulate drug delivery. Int. J. Biol. Macromol. 2015, 72, 495–501. [Google Scholar] [CrossRef]
- Erum, A.; Bashir, S.; Saghir, S.; Tulain, U.R.; Saleem, U.; Nasir, M.; Kanwal, F.; Hayat malik, M.N. Acute toxicity studies of a novel excipient arabinoxylan isolated from Ispaghula (Plantago ovata) husk. Drug Chem. Toxicol. 2015, 38, 300–305. [Google Scholar] [CrossRef]
- Saghir, S.; Iqbal, M.S.; Koschella, A.; Heinze, T. Ethylation of arabinoxylan from Ispaghula (Plantago ovata) seed husk. Carbohydr. Polym. 2009, 77, 125–130. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Akbar, J.; Hussain, M.A.; Saghir, S.; Sher, M. Evaluation of hot-water extracted arabinoxylans from ispaghula seeds as drug carriers. Carbohydr. Polym. 2011, 83, 1218–1225. [Google Scholar] [CrossRef]
- Saghir, S.; Iqbal, M.S.; Hussain, M.A.; Koschella, A.; Heinze, T. Structure characterization and carboxymethylation of arabinoxylan isolated from Ispaghula (Plantago ovata) seed husk. Carbohydr. Polym. 2008, 74, 309–317. [Google Scholar] [CrossRef]
- Rezvanain, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Stevens, H.; Auffret, A.; Humphrey, M.; Eccleston, G. Lyophilised wafers as a drug delivery system for wound healing containing methylcellulose as a viscosity modifier. Int. J. Pharm. 2005, 289, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B.; Gadzala-Kopciuch, R. Gentamicin release from chitosan and collagen composites. J. Drug Deliv. Sci. Technol. 2016, 35, 353–359. [Google Scholar] [CrossRef]
- Kachel-Jakubowska, M.; Matwijczuk, A.; Gagoś, M. Analysis of the physicochemical properties of post-manufacturing waste derived from production of methyl esters from rapeseed oil. Int. Agrophys. 2017, 31, 175–182. [Google Scholar] [CrossRef]
- Perotti, G.F.; Kijchavengkul, T.; Auras, R.A.; Constantino, V.R. Nanocomposites Based on Cassava Starch and Chitosan-Modified Clay: Physico-Mechanical Properties and Biodegradability in Simulated Compost Soil. J. Braz. Chem. Soc. 2017, 28, 649–658. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Akbar, J.; Saghir, S.; Karim, A.; Koschella, A.; Heinze, T.; Sher, M. Thermal studies of plant carbohydrate polymer hydrogels. Carbohydr. Polym. 2011, 86, 1775–1783. [Google Scholar] [CrossRef]
- Almazrouei, M.; Samad, T.E.; Janajreh, I. Thermogravimetric Kinetics and High Fidelity Analysis of Crude Glycerol. Energy Procedia 2017, 142, 1699–1705. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Erum, A.; Bashir, S.; Saghir, S. Modified and unmodified arabinoxylans from Plantago ovata husk: Novel excipients with antimicrobial potential. Bangladesh J. Pharmacol. 2015, 10, 765–769. [Google Scholar] [CrossRef] [Green Version]
- ISO I. 10993–5: 2009 Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009.







| Film Code | Arabinoxylan (% w/v) | Glycerol (% w/v) | Gentamicin (% w/v) | Water (q·s·mL) | Gel Weight (g/film) |
|---|---|---|---|---|---|
| AXF | 2 | – | – | 100 | 33 |
| AXF21 | 2 | 1 | – | 100 | 33 |
| AXF1 | 1 | 2 | – | 100 | 33 |
| AXF1.5 | 1.5 | 2 | – | 100 | 33 |
| AXF2 | 2 | 2 | – | 100 | 33 |
| AXF2.5 | 2.5 | 2 | – | 100 | 33 |
| AXF3 | 3 | 2 | – | 100 | 33 |
| AXF3.5 | 3.5 | 2 | – | 100 | 33 |
| AXDF2 | 2 | 2 | 0.1 | 100 | 33 |
| AXDF2.5 | 2.5 | 2 | 0.1 | 100 | 33 |
| AXDF3 | 3 | 2 | 0.1 | 100 | 33 |
| Film Code | Peelablity | Transparency | Bubbles | Flexibility | Interpretation |
|---|---|---|---|---|---|
| AXF | × | √ | √ | × | Rejected: thin, brittle, non-peelable, non-flexible films |
| AXF21 | × | √ | × | × | Rejected: rigid films, difficult to peel |
| AXF1 | × | √ | × | √ | Rejected: thin, non-peelable, torn during peeling |
| AXF1.5 | × | √ | × | √ | Rejected: thin, non-peelable, torn during peeling |
| AXF2 | √ | √ | × | √ | Selected: transparent, easily peelable, and flexible films |
| AXF2.5 | √ | √ | × | √ | Selected: transparent, easily peelable, and flexible films |
| AXF3 | √ | √ | × | √ | Selected: transparent, easily peelable, and flexible films |
| AXF3.5 | √ | × | √ | √ | Rejected: form viscous gel, difficult to pour and stir, uneven thickness, less transparent, bubbles |
| Films Code | Thickness (mm) | Solvent Loss (%) | Tensile Strength (N/mm2) | Elongation at Break (%) | WVTR (g/m2/day) |
|---|---|---|---|---|---|
| AXF2 | 0.309 ± 0.007 | 96.5 ± 1.4 | Film damaged | 1915 ± 135 | |
| AXF2.5 | 0.312 ± 0.008 | 96.1 ± 1.7 | 3.17 ± 0.14 # | 95.1 ± 6.3 # | 1747 ± 121 |
| AXF3 | 0.331 ± 0.005 # | 95.2 ± 2.1 | 3.75 ± 0.21 # | 83.7 ± 4.6 # | 1757 ± 130 |
| AXDF2 | 0.333 ± 0.007 *× | 94.9 ± 2.3 | Film damaged | 1842 ± 170 | |
| AXDF2.5 | 0.354 ± 0.004 *× | 92.3 ± 1.3 | 3.31 ± 0.17 × | 97.3 ± 3.4 × | 1631 ± 130 |
| AXDF3 | 0.356 ± 0.006 *× | 91.7 ± 2.2 | 3.79 ± 0.19 × | 84.9 ± 5.7 × | 1665 ± 155 |
| Films | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | Hixson–Crowell | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K0 | R2 | K1 | R2 | K | R2 | K | n | R2 | K | |
| AXFD2.5 | 0.614 | 2.246 | 0.799 | −0.037 | 0.819 | 14.63 | 0.911 | 1.565 | 0.511 | 0.615 | −0.749 |
| AXFD3 | 0.685 | 2.901 | 0.896 | −0.044 | 0.875 | 18.50 | 0.979 | 1.405 | 0.651 | 0.685 | −0.967 |
| Sample | S. aureus (ZOI mm) | P. aeruginosa (ZOI mm) | E. coli (ZOI mm) |
|---|---|---|---|
| Blank Disk | 9.3 ± 1.6 | 6.6 ± 0.8 | 6.4 ± 2.3 |
| Blank Film | 20.6 ± 1.2 * | 11.2 ± 0.8 * | 10.9 ± 1.2 |
| GM Standard | 31.8 ± 1.5 *# | 27.5 ± 1.6 *# | 21.9 ± 2.1 *# |
| AXFD2 | 34.7 ± 1.7 *# | 20.1 ± 1.1 *#× | 21.7 ± 2.2 *# |
| AXFD2.5 | 31.2 ± 1.9 *# | 19.9 ± 1.2 *#× | 21.1 ± 1.5 *# |
| AXFD3 | 30.3 ± 1.3 *# | 23.3 ± 1.4 *# | 21.1 ± 1.7 *# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Tayyeb, D.; Ali, I.; K. Alruwaili, N.; Ahmad, W.; ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. https://doi.org/10.3390/polym12030548
Ahmad N, Tayyeb D, Ali I, K. Alruwaili N, Ahmad W, ur Rehman A, Khan AH, Iqbal MS. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers. 2020; 12(3):548. https://doi.org/10.3390/polym12030548
Chicago/Turabian StyleAhmad, Naveed, Danial Tayyeb, Imran Ali, Nabil K. Alruwaili, Waqas Ahmad, Atta ur Rehman, Abdul Haleem Khan, and Mohammad Saeed Iqbal. 2020. "Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application" Polymers 12, no. 3: 548. https://doi.org/10.3390/polym12030548
APA StyleAhmad, N., Tayyeb, D., Ali, I., K. Alruwaili, N., Ahmad, W., ur Rehman, A., Khan, A. H., & Iqbal, M. S. (2020). Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers, 12(3), 548. https://doi.org/10.3390/polym12030548