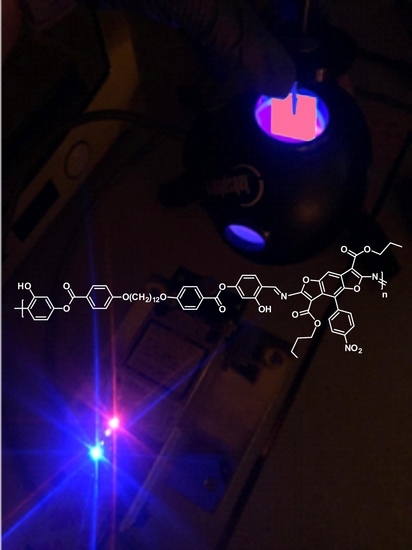
Novel Solid-State Emissive Polymers and Polymeric Blends from a T-Shaped Benzodifuran Scaffold: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Preparation of Thin Films for Optical Characterizations and PLQY Setup
2.4. X-ray Analysis
2.5. Synthesis of the Polymers
2.6. Synthesis of the Models
3. Results and Discussion
3.1. Polymers and Model Compounds
3.2. PVK Blends
3.3. Single Crystal X-ray Analysis of BDF–NO2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weder, C. Mechanoresponsive Materials. J. Mater. Chem. 2011, 21, 8235. [Google Scholar] [CrossRef]
- Ciardelli, F.; Ruggeri, G.; Pucci, A. Dye-containing polymers: Methods for preparation of mechanochromic materials. Chem. Soc. Rev. 2013, 42, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, K.; Feng, G.; Li, M.; Yang, Z.; Liu, B.; Tang, B.Z. Bright and Photostable Organic Fluorescent Dots with Aggregation-Induced Emission Characteristics for Noninvasive Long-Term Cell Imaging. Adv. Funct. Mater. 2013, 24, 635–643. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Mechanically induced luminescence changes in molecular assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, C.; Gao, D.; Tang, X.; Dong, X.; Lin, X.; Wang, Y.; Wang, X.; Wang, L.; Lee, H.H.; et al. Drastic photoluminescence modulation of an organic molecular crystal with high pressure. Mater. Chem. Front. 2019, 3, 1510–1517. [Google Scholar] [CrossRef]
- Hou, X.; Ke, C.; Bruns, C.; McGonigal, P.R.; Pettman, R.B.; Stoddart, J.F. Tunable solid-state fluorescent materials for supramolecular encryption. Nat. Commun. 2015, 6, 6884. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Yang, Z.; Lam, J.W.Y.; Sung, H.H.Y.; Xie, N.; Chen, S.; Su, H.; Gao, M.; Williams, I.D.; Wong, K.S.; et al. Benzothiazolium-functionalized tetraphenylethene: An AIE luminogen with tunable solid-state emission. Chem. Commun. 2012, 48, 8637–8639. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Li, J.; Li, C.; Heng, L.; Dong, Y.Q.; Liu, Z.; Bo, Z.; Tang, B.Z. Reversible Switching of the Emission of Diphenyldibenzofulvenes by Thermal and Mechanical Stimuli. Adv. Mater. 2011, 23, 3261–3265. [Google Scholar] [CrossRef]
- Caruso, U.; Diana, R.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Facile synthesis of new Pd(II) and Cu(II) based metallomesogens from ligands containing thiophene rings. Inorg. Chem. Commun. 2009, 12, 1135–1138. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, X.; Xu, Y.; Liu, H.; Zhang, S.-T.; Yang, B.; Ma, Y. Enhanced deep-red emission in donor-acceptor molecular architecture: The role of ancillary acceptor of cyanophenyl. Chin. Chem. Lett. 2019, 30, 1947–1950. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Piotto, S. AIE/ACQ Effects in Two DR/NIR Emitters: A Structural and DFT Comparative Analysis. Molecules 2018, 23, 1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalapati, S.; Jin, E.; Addicoat, M.; Heine, T.; Jiang, D. Highly Emissive Covalent Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 5797–5800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, W.; Hao, X.; Redshaw, C.; Chen, L.; Sun, W.-H. 6-Benzhydryl-4-methyl-2-(1H-benzoimidazol-2-yl)phenol ligands and their zinc complexes: Syntheses, characterization and photoluminescence behavior. Inorg. Chim. Acta 2012, 392, 345–353. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks. Polymers 2019, 11, 1379. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Liu, Y.; Li, Y.; Feng, Q.; Hou, H.; Tang, B.Z. 2,5-bis(4-alkoxycarbonylphenyl)-1,4-diaryl-1,4-dihydropyrrolo[3,2-b]pyrrole (AAPP) AIEgens: Tunable RIR and TICT characteristics and their multifunctional applications. Chem. Sci. 2017, 8, 7258–7267. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Leung, N.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Feng, Z.; Mohapatra, S.; Klimko, P.G.; Hellberg, M.R.; May, J.A.; Kelly, C.; Williams, G.; McLaughlin, M.A.; Sharif, N.A. Novel benzodifuran analogs as potent 5-HT2A receptor agonists with ocular hypotensive activity. Bioorganic Med. Chem. Lett. 2007, 17, 2998–3002. [Google Scholar] [CrossRef]
- Carella, A.; Roviello, V.; Iannitti, R.; Palumbo, R.; La Manna, S.; Marasco, D.; Trifuoggi, M.; Diana, R.; Roviello, V. Evaluating the biological properties of synthetic 4-nitrophenyl functionalized benzofuran derivatives with telomeric DNA binding and antiproliferative activities. Int. J. Biol. Macromol. 2019, 121, 77–88. [Google Scholar] [CrossRef]
- Migalska-Zalas, A.; El Korchi, K.; Chtouki, T. Enhanced nonlinear optical properties due to electronic delocalization in conjugated benzodifuran derivatives. Opt. Quantum Electron. 2018, 50, 389. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Chen, Y.; Chen, W.; Qiao, S.; Wen, S.; Liu, Q.; Zhu, D.; Sun, M.; Yang, R. Development of New Two-Dimensional Small Molecules Based on Benzodifuran for Efficient Organic Solar Cells. Chem. Asian J. 2014, 9, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Pron, A.; Gawrys, P.; Zagorska, M.; Djurado, D.; Demadrille, R. Electroactive materials for organic electronics: Preparation strategies, structural aspects and characterization techniques. Chem. Soc. Rev. 2010, 39, 2577. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bäuerle, P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Angew. Chem. Int. Ed. 2012, 51, 2020–2067. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, V.; Roviello, G.; Tingoli, M.; Tuzi, A. Synthesis, structure and reactivity of amino-benzodifurane derivatives. Comptes Rendus Chim. 2009, 12, 622–634. [Google Scholar] [CrossRef]
- Mbarek, M.; Massuyeau, F.; Wery, J.; Faulques, E.; Alimi, K.; Duvail, J.-L. New copolymer of poly(N-vinylcarbazole) and poly(p-phenylenevinylene) for optoelectronic devices. J. Appl. Polym. Sci. 2013, 130, 2839–2847. [Google Scholar] [CrossRef]
- D’Angelo, P.; Barra, M.; Cassinese, A.; Maglione, M.; Vacca, P.; Minarini, C.; Rubino, A. Electrical transport properties characterization of PVK (poly N-vinylcarbazole) for electroluminescent devices applications. Solid-State Electron. 2007, 51, 123–129. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Sirigu, A. Networks from Liquid Crystalline Segmented Chain Polymers. Macromolecules 1994, 27, 3513–3519. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS (Version 2.03); University of Göttingen: Göttingen, Germany, 2002. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP -III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Borbone, F.; Carella, A.; Caruso, U.; Roviello, G.; Tuzi, A.; Dardano, P.; Lettieri, S.; Maddalena, P.; Barsella, A. Large Second-Order NLO Activity in Poly(4-vinylpyridine) Grafted with PdII and CuII Chromophoric Complexes with Tridentate Bent Ligands Containing Heterocycles. Eur. J. Inorg. Chem. 2008, 2008, 1846–1853. [Google Scholar] [CrossRef]
- Gupta, R.K.; Achalkumar, A.S. Perylene-Based Liquid Crystals as Materials for Organic Electronics Applications. Langmuir 2018, 35, 2455–2479. [Google Scholar] [CrossRef]
- Panunzi, B.; Concilio, S.; Diana, R.; Shikler, R.; Nabha-Barnea, S.; Piotto, S.; Sessa, L.; Tuzi, A.; Caruso, U. Photophysical Properties of Luminescent Zinc(II)-Pyridinyloxadiazole Complexes and their Glassy Self-Assembly Networks. Eur. J. Inorg. Chem. 2018, 2018, 2709–2716. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Di Palma, S.; Fusco, S.; Nabha-Barnea, S.; Panunzi, B.; Shikler, R. High Solid State Photoluminescence Quantum Yields and Effective Color Tuning in Polyvinylpyridine Based Zinc(II) Metallopolymers. Macromol. Chem. Phys. 2015, 216, 1516–1522. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha-Barnea, S.; Tuzi, A.; Caruso, U.; Piotto, S. Solid-State Highly Efficient DR Mono and Poly-dicyano-phenylenevinylene Fluorophores. Molecules 2018, 23, 1505. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha-Barnea, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Panunzi, B.; Borbone, F.; Capobianco, A.; Concilio, S.; Diana, R.; Peluso, A.; Piotto, S.; Tuzi, A.; Velardo, A.; Caruso, U. Synthesis, spectroscopic properties and DFT calculations of a novel multipolar azo dye and its zinc(II) complex. Inorg. Chem. Commun. 2017, 84, 103–108. [Google Scholar] [CrossRef]
- Yi, C.-L.; Ko, C.-L.; Yeh, T.-C.; Chen, C.-Y.; Chen, Y.-S.; Chen, D.-G.; Chou, P.-T.; Hung, W.-Y.; Wong, K.-T. Harnessing a New Co-Host System and Low Concentration of New TADF Emitters Equipped with Trifluoromethyl- and Cyano-Substituted Benzene as Core for High-Efficiency Blue OLEDs. ACS Appl. Mater. Interfaces 2019, 12, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Luo, Z.-H.; Huan, I.H.; Chen, Y.-H.; Lim, T.-S. Rationalize the roles of electron donating-withdrawing groups in the impacts on solvatochromism, nonlinear optics, and electroluminescence devices. Dyes Pigment. 2020, 175, 108143. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Lin, F.; Li, H.; Fan, Y.; Zhang, N. Highly Conductive Transparent Organic Electrodes with Multilayer Structures for Rigid and Flexible Optoelectronics. Sci. Rep. 2015, 5, 10569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diana, R.; Tuzi, A.; Panunzi, B.; Carella, A.; Caruso, U. Crystal structures of butyl 2-amino-5-hy-droxy-4-(4-nitro-phen-yl)benzo-furan-3-carboxyl-ate and 2-meth-oxy-ethyl 2-amino-5-hy-droxy-4-(4-nitro-phen-yl)benzo-furan-3-carboxyl-ate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2019, 75, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Fang, C. Nitration of Tyrosine Channels Photoenergy through a Conical Intersection in Water. J. Phys. Chem. B 2019, 123, 4915–4928. [Google Scholar] [CrossRef]
- Ernst, H.A.; Wolf, T.J.A.; Schalk, O.; González-García, N.; Boguslavskiy, A.E.; Stolow, A.; Olzmann, M.; Unterreiner, A.-N. Ultrafast Dynamics of o-Nitrophenol: An Experimental and Theoretical Study. J. Phys. Chem. A 2015, 119, 9225–9235. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 17.5; University of Western Australia: Perth, Australia, 2017. [Google Scholar]








| CCDC Number | 1986514 |
|---|---|
| Empirical Formula | C26H27N3O8 |
| Formula Weight | 509.50 |
| Temperature (K) | 173(2) |
| Wavelength (Å) | 0.71073 |
| Crystal System (Å) | Monoclinic |
| Space Group | C2/c |
| a (Å) | 25.644(3) |
| b (Å) | 12.1350(16) |
| c (Å) | 16.8623(16) |
| α (°) | 90 |
| β (°) | 112.021(7) |
| γ (°) | 90 |
| Volume (Å3) | 4864.6(10) |
| Z | 8 |
| Dcalc (Mg/m3) | 1.391 |
| μ (mm−1) | 0.104 |
| F(000) | 2144 |
| Crystal Size (mm) | 0.50 × 0.25 × 0.10 |
| θ Range (°) | 2.935 to 27.495 |
| Reflections Collected/Unique | 17016/5461 [R(int) = 0.0605] |
| Refinement Method | Full-matrix least-squares on F2 |
| Data/Restraints/Parameters | 5461/103/386 |
| Goodness-of-fit on F2 | 1.038 |
| Final R Indices [I > 2 sigma(I)] | R1 = 0.0633, wR2 = 0.1304 |
| R Indices (all data) | R1 = 0.1331, wR2 = 0.1583 |
| Largest diff. Peak and Hole (eA−3) | 0.638 and −0.385 |
| D–H…A | d(D–H) | d(H…A) | d(D…A) | <(DHA) |
|---|---|---|---|---|
| C20–H15…O8i | 0.95 | 2.66 | 3.566(4) | 160.5 |
| C20–H15…O8i | 0.95 | 2.66 | 3.566(4) | 160.5 |
| C12–H18A…O7ii | 0.99 | 2.54 | 3.356(4) | 139.2 |
| N1–H1B…O4 | 0.82(3) | 2.38(3) | 2.917(3) | 123(2) |
| N1–H1B…O6iii | 0.82(3) | 2.19(3) | 2.902(3) | 146(3) |
| N2–H2A…O7iv | 0.86(3) | 2.57(3) | 3.378(4) | 156(3) |
| N2–H2A…O8iv | 0.86(3) | 2.42(3) | 3.213(3) | 154(3) |
| N2–H2B…O4v | 0.85(3) | 2.32(3) | 3.059(3) | 145(3) |
| N2–H2B…O6 | 0.85(3) | 2.22(3) | 2.777(3) | 124(3) |
| Sample | λab.sol (nm)[a] | λem.sol (nm)[b] | λab.film (nm)[c] | λem.film (nm)[d] | CIE Coord.[e] | Stokes Shift (nm)[f] | PLQY%[g] |
|---|---|---|---|---|---|---|---|
| M1 | 456 | 528 | 470 | 607 | 0.40; 0.37 | 137 | 23 ± 0.2 |
| M2 | 430 | 504 | 430 | 550 | 0.39; 0.46 | 120 | 1.5 ± 0.2 |
| P1 | 457 | 512 | 453 | 609 | 0.42; 0.33 | 156 | 22 ± 0.2 |
| P2 | 438 | 515 | 435 | 565 | 0.36; 0.41 | 130 | 2.0 ± 0.2 |
| M1-PVK | - | - | 443 | 582 | 0.40; 0.35 | 139 | 14 ± 0.2 |
| M2-PVK | - | - | 411 | 522 | 0.39; 0.45 | 111 | 1.5 ± 0.2 |
| Group | Chemical Shift (ppm) | |||
|---|---|---|---|---|
| M1 | M2 | P1 | P2 | |
| OCH3 | 3.92 | 3.92 | ||
| OCH2 (of dialdehyde) | 3.99 | 4.03 | ||
| OCH2 (of BDF-NO2) | 4.56 | 4.54 | 4.61 | 4.30 |
| NH2 | 6.56 | 6.56 | ||
| CN=N | 10.00 | 9.97 | 10.16 | 10.07 |
| OH | 13.05 | |||
| Compound | Mp/Tg (°C)[a] | Ti (°C)[b] | Td (°C)[c] |
|---|---|---|---|
| M1 | 261 | - | 288 |
| M2 | 289 | - | 296 |
| P1 | 129 | 188 | 330 |
| P2 | 120 | 130 | 325 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, U.; Diana, R.; Tuzi, A.; Panunzi, B. Novel Solid-State Emissive Polymers and Polymeric Blends from a T-Shaped Benzodifuran Scaffold: A Comparative Study. Polymers 2020, 12, 718. https://doi.org/10.3390/polym12030718
Caruso U, Diana R, Tuzi A, Panunzi B. Novel Solid-State Emissive Polymers and Polymeric Blends from a T-Shaped Benzodifuran Scaffold: A Comparative Study. Polymers. 2020; 12(3):718. https://doi.org/10.3390/polym12030718
Chicago/Turabian StyleCaruso, Ugo, Rosita Diana, Angela Tuzi, and Barbara Panunzi. 2020. "Novel Solid-State Emissive Polymers and Polymeric Blends from a T-Shaped Benzodifuran Scaffold: A Comparative Study" Polymers 12, no. 3: 718. https://doi.org/10.3390/polym12030718
APA StyleCaruso, U., Diana, R., Tuzi, A., & Panunzi, B. (2020). Novel Solid-State Emissive Polymers and Polymeric Blends from a T-Shaped Benzodifuran Scaffold: A Comparative Study. Polymers, 12(3), 718. https://doi.org/10.3390/polym12030718