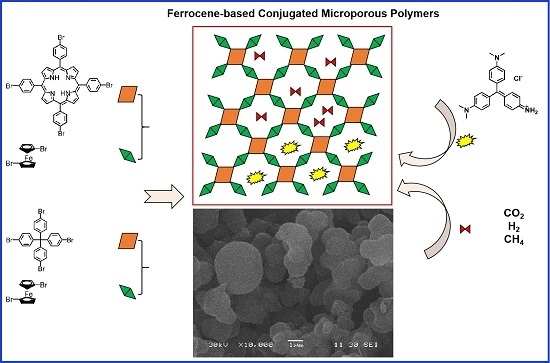
Ferrocene-Based Conjugated Microporous Polymers Derived from Yamamoto Coupling for Gas Storage and Dye Removal
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of FcCMPs
2.3. Characterization
3. Results and Discussion
3.1. Chemical Structure of FcCMPs
3.2. Properties of FcCMPs
3.3. Pore structure of FcCMPs
3.4. Gas Uptakes of FcCMPs
3.5. Methyl Violet Adsorption of FcCMPs
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cooper, A.I. Conjugated microporous polymers. Adv. Mater. 2010, 21, 1291–1295. [Google Scholar] [CrossRef]
- Li, G.; Liu, Q.Q.; Liao, B.; Chen, L.J.; Zhou, H.; Zhou, Z.H.; Xia, B.J.; Huang, J.; Liu, B. Synthesis of novel ferrocene-based conjugated microporous polymers with intrinsic magnetism. Eur. Polym. J. 2017, 93, 556–560. [Google Scholar] [CrossRef]
- Bezzu, C.G.; Carta, M.; Tonkins, A.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; Mckeown, N.B. A spirobifluorene-based polymer of intrinsic microporosity with improved performance for gas separation. Adv. Mater. 2012, 24, 5930–5933. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Park, H.B.; Robertson, G.P.; Dal-Cin, M.M.; Visser, T.; Scoles, L.; Guiver, M.D. Polymer nanosieve membranes for CO2-capture applications. Nat. Mater. 2011, 10, 372–375. [Google Scholar] [CrossRef]
- Dawson, R.; Adams, D.J.; Cooper, A.I. Chemical tuning of CO2 sorption in robust nanoporous organic polymers. Chem. Sci. 2011, 2, 1173–1177. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet, J.M.; Svec, F. Nanoporous polymers for hydrogen storage. Small 2009, 5, 1098–1111. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ghasimi, S.; Landfester, K.; Zhang, K. Molecular structural design of conjugated microporous poly(benzooxadiazole) networks for enhanced photocatalytic activity with visible light. Adv. Mater. 2015, 27, 6265–6270. [Google Scholar] [CrossRef]
- Wang, Z.J.; Garth, K.; Ghasimi, S.; Landfester, K.; Zhang, K.A. Conjugated microporous poly(Benzochalcogenadiazole)s for photocatalytic oxidative coupling of amines under visible light. ChemSusChem 2015, 8, 3459–3464. [Google Scholar] [CrossRef]
- Zhang, W.J.; Tang, J.T.; Yu, W.; Huang, Q.; Fu, Y.; Kuang, G.C.; Pan, C.Y.; Yu, G.P. Visible light-driven C-3 functionalization of indoles over conjugated microporous polymers. ACS Catal. 2018, 8, 8084–8091. [Google Scholar] [CrossRef]
- Xu, Y.; Nagai, A.; Jiang, D. Core-shell conjugated microporous polymers: A new strategy for exploring color-tunable and -controllable light emissions. Chem. Commun. 2013, 49, 1591–1593. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Tang, Z.; Wu, M.D.; Liao, B.; Zhou, H.; Ou, B.L.; Yu, G.P.; Zhou, Z.H.; Li, X.J. Novel ferrocene-based nanoporous organic polymers for clean energy application. RSC Adv. 2015, 5, 8933–8977. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, S.; Mason, A.; Reprogle, B.; Liu, D.J.; Yu, L. Nanoporous porphyrin polymers for gas storage and separation. Macromolecules 2012, 45, 7413–7419. [Google Scholar] [CrossRef]
- Kiskan, B.; Weber, J. Versatile postmodification of conjugated microporous polymers using thiol-yne chemistry. ACS Macro Lett. 2012, 1, 37–40. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Cao, D.P.; Wang, W.C.; Yang, W.T.; Han, B.Y.; Lu, J.M. Postsynthetic lithium modification of covalent-organic polymers for enhancing hydrogen and carbon dioxide storage. J. Phys. Chem. C 2012, 116, 5974–5980. [Google Scholar] [CrossRef]
- Ma, H.P.; Ren, H.; Zou, X.Q.; Meng, S.; Su, F.X.; Zhu, G.S. Post-metalation of porous aromatic frameworks for highly efficient carbon capture from CO2+N2 and CH4+N2 mixtures. Polym. Chem. 2014, 5, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Sun, L.; Li, G.; Shang, J.; Yang, R.X.; Deng, W.Q. Methyllithium-doped naphthyl-containing conjugated microporous polymer with enhanced hydrogen storage performance. Chem. Eur. J. 2016, 22, 7944–7949. [Google Scholar] [CrossRef]
- Fu, X.; Zang, Y.D.; Gu, S.; Zhu, Y.L.; Yu, G.P.; Pan, C.Y.; Wang, Z.G.; Hu, Y.H. Metal microporous aromatic polymers with improved performance for small gas storage. Chem. Eur. J. 2015, 21, 3357–13363. [Google Scholar] [CrossRef]
- Li, A.; Lu, R.F.; Wang, Y.; Wang, X.; Han, K.L.; Deng, W.Q. Lithium-doped conjugated microporous polymers for reversible hydrogen storage. Angew. Chem. Int. Ed. 2010, 49, 3330–3333. [Google Scholar] [CrossRef]
- Srinivasu, K.; Ghosh, S.K. Hydrogen adsorption in lithium decorated conjugated microporous polymers: A DFT investigation. RSC Adv. 2014, 4, 4170–4176. [Google Scholar] [CrossRef]
- Li, G.; Liu, Q.Q.; Xia, B.J.; Huang, J.; Li, S.Z.; Guang, Y.Z.; Zhou, H.; Liao, B.; Zhou, Z.H.; Liu, B. Synthesis of stable metal-containing porous organic polymers for gas storage. Eur. Polym. J. 2017, 91, 242–247. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, D.; Zhao, D.; Schilling, C.I.; Plietzsch, O.; Muller, T.; Bräse, S.; Guenther, J.; Blümel, J.; Krishna, R.; et al. Porous polymer networks: Synthesis, porosity, and applications in gas storage/separation. Chem. Mater. 2010, 22, 5964–5972. [Google Scholar] [CrossRef]
- Liu, X.M.; Sigen, A.; Zhang, Y.W.; Luo, X.L.; Xia, H.; Li, H.; Mu, Y. A porphyrin-linked conjugated microporous polymer with selective carbon dioxide adsorption and heterogeneous organocatalytic performances. RSC Adv. 2014, 4, 6447–6453. [Google Scholar] [CrossRef]
- Okada, Y.; Oguri, K.; Sakamoto, K.; Miyako, Y.; Hayashi, T. Spectral assignments and reference data. Magn. Reson. Chem. 2002, 40, 795–796. [Google Scholar] [CrossRef]
- Gassman, P.G.; Macomber, D.W.; Hershberger, J.W. Evaluation by ESCA of the electronic effect of methyl substitution on the cyclopentadienyl ligand. A study of titanocenes, zirconocenes, hafnocenes, and ferrocenes. Organometallics 1983, 2, 1470–1472. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.S.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Rabbani, M.; Sekizkardes, A.; Kahveci, Z.; Reich, T.; Ding, R.; El-Kaderi, H. A 2D mesoporous imine-linked covalent organic framework for high pressure gas storage applications. Chem. Eur. J. 2013, 19, 3324–3328. [Google Scholar] [CrossRef]
- Kahveci, Z.; Islamoglu, T.; Shar, G.; Ding, R.; El-Kaderi, H.M. Targeted synthesis of a mesoporous triptycene-derived covalent organic framework. CrystEngComm 2013, 15, 1524–1527. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, H.; Sigen, A.; Xia, H.; Mu, Y. Triarylboron-based fluorescent conjugated microporous polymers. RSC Adv. 2013, 3, 21267–21270. [Google Scholar] [CrossRef]
- Ren, S.J.; Dawson, R.; Laybourn, A.; Jiang, J.X.; Khimyak, Y.; Adams, D.J.; Cooper, A.I. Functional conjugated microporous polymers: From 1,3,5-benzene to 1,3,5-triazine. Polym. Chem. 2012, 3, 928–934. [Google Scholar] [CrossRef]
- Sun, X.; Qi, Y.; Li, J.; Wang, W.; Ma, Q.; Liang, J. Ferrocene-linked porous organic polymers for carbon dioxide and hydrogen sorption. J. Organometal. Chem. 2018, 859, 117–123. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.P.; Zhu, J.H.; Han, B.H. Mesoporous conjugated polycarbazole with high porosity via structure tuning. Macromolecules 2014, 47, 5926–5931. [Google Scholar] [CrossRef]
- Jackson, K.T.; Rabbani, M.G.; Reich, T.E.; El-Kaderi, H.M. Synthesis of highly porous borazine-linked polymers and their application to H2, CO2, and CH4 storage. Polym. Chem. 2011, 2, 2775–2777. [Google Scholar] [CrossRef]
- Martín, C.F.; Stkel, E.; Clowes, R.; Adams, D.J.; Cooper, A.I.; Pis, J.J.; Rubiera, F.; Pevida, C. Hypercrosslinked organic polymer networks as potential adsorbents for pre-combustion CO2 capture. J. Mater. Chem. 2011, 21, 5475–5483. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.Q. Monodisperse polystyrene nanospheres with ultra-high surface area and their application in hydrogen storage. Macromol. Chem. Phys. 2010, 211, 1012–1017. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, D.; Sculley, J.; Zhao, D.; Krishna, R.; Zhou, H.C. Sulfonate-grafted porous polymer networks for preferential CO2 adsorption at low pressure. J. Am. Chem. Soc. 2011, 133, 18126–18129. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, Z.G. Microporous polyimides with uniform pores for adsorption and separation of CO2 gas and organic vapors. Macromolecules 2013, 46, 3058–3066. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Pan, C.Y. A novel route to treat wastewater containing cationic dyes. Sep. Sci. Technol. 2012, 47, 630–635. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Wang, L.; Xiao, A.G.; Gao, J.M.; Ding, W.B.; Yu, H.J.; Huo, J.; Ericson, M. Templated preparation of porous magnetic microspheres and their application in removal of cationic dyes from wastewater. J. Hazard. Mater. 2010, 181, 586–592. [Google Scholar] [CrossRef]








| SBET(m2/g) | Smicro(m2/g) | Vmicro(m3/g) | Vtotal(m3/g) | Vmicro/ Vtotal | |
|---|---|---|---|---|---|
| FcCMP-1 | 638 | 475 | 0.19 | 0.29 | 65.5% |
| FcCMP-2 | 422 | 306 | 0.14 | 0.23 | 60.9% |
| H2 Uptake | CO2 Uptake | CH4 Uptake | S(CO2/N2) | Qst (CO2) (KJ/mol) | Qst (CH4) (KJ/mol) | |
|---|---|---|---|---|---|---|
| Temp. (K) | 77 | 273 | 273 | 273 | — | — |
| FcCMP-1 | 1.1 wt % | 9.6 wt % | 1.1 wt % | 51.5 | 26.5 | 18.6 |
| 5.5 mmol/g | 2.2 mmol/g | 0.69 mmol/g | ||||
| FcCMP-2 | 0.7 wt % | 7.1 wt % | 0.9 wt % | 2.8 | 30.3 | 27.9 |
| 3.5 mmol/g | 1.6 mmol/g | 0.64 mmol/g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Su, H.; Guo, Y.; Liu, H.; Liao, B.; Amin, A.M.; Liu, Q. Ferrocene-Based Conjugated Microporous Polymers Derived from Yamamoto Coupling for Gas Storage and Dye Removal. Polymers 2020, 12, 719. https://doi.org/10.3390/polym12030719
Tan Z, Su H, Guo Y, Liu H, Liao B, Amin AM, Liu Q. Ferrocene-Based Conjugated Microporous Polymers Derived from Yamamoto Coupling for Gas Storage and Dye Removal. Polymers. 2020; 12(3):719. https://doi.org/10.3390/polym12030719
Chicago/Turabian StyleTan, Zhiqiang, Huimin Su, Yiwen Guo, Huan Liu, Bo Liao, Abid Muhammad Amin, and Qingquan Liu. 2020. "Ferrocene-Based Conjugated Microporous Polymers Derived from Yamamoto Coupling for Gas Storage and Dye Removal" Polymers 12, no. 3: 719. https://doi.org/10.3390/polym12030719
APA StyleTan, Z., Su, H., Guo, Y., Liu, H., Liao, B., Amin, A. M., & Liu, Q. (2020). Ferrocene-Based Conjugated Microporous Polymers Derived from Yamamoto Coupling for Gas Storage and Dye Removal. Polymers, 12(3), 719. https://doi.org/10.3390/polym12030719