Mono-Dispersed Microspheres Locally Assembled on Porous Substrates Formed through a Microemulsion Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
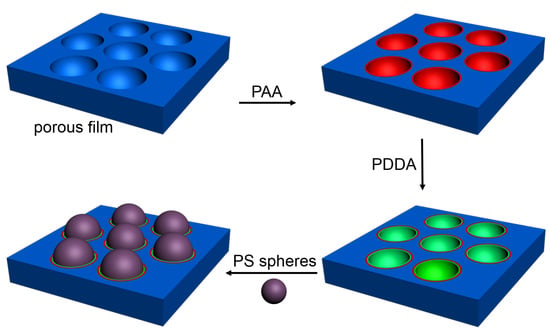
2.2. Preparation of Porous Film
2.3. Assembly of PAA and PDDA into the Porous Cavities
2.4. The Assembly of PS Microspheres into the Cavities
2.5. Measurements
3. Results and Discussion
3.1. Preparation and Structural Characterization of Polymer Porous Films
3.2. Selective Assembly of PAA and PDDA into Patterned Cavities
3.3. The Build of Microsphere-Based Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fan, X.Y.; Rui, Y.C.; Han, X.F.; Yang, J.X.; Wang, Y.Q.; Zhang, Q.D. Spray-coated monodispersed SnO2 microsphere films as scaffold layers for efficient mesoscopic perovskite solar cells. J. Power Sources 2020. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, W.G.; Hu, T.; Chen, P.; Lan, T.; Li, P.; Li, Y.; Mi, B.X.; Ma, Y.W. Controlling Electrode Spacing by Polystyrene Microsphere Spacers for Highly Stable and Flexible Transparent Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 5885–5891. [Google Scholar] [CrossRef]
- Zhang, X.H.; Que, W.X.; Gao, T.X. Monolayer Polystyrene Micro-Spheres Array Master Derived by Spin-Coating Method for UV Nanoimprint. J. Nanosci. Nanotechnol. 2012, 12, 6538–6542. [Google Scholar] [CrossRef]
- Li, Y.F.; Sun, Z.Q.; Zhang, J.H.; Zhang, K.; Wang, Y.F.; Wang, Z.H.; Chen, X.L.; Zhu, S.J.; Yang, B. Polystyrene@TiO2 core–shell microsphere colloidal crystals and nonspherical macro-porous materials. J. Colloid Interfaces Sci. 2008, 325, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Domonkos, M.; Varga, M.; Ondič, L.; Gajdošová, L.; Kromka, A. Microsphere lithography for scalable polycrystalline diamond-based near-infrared photonic crystals fabrication. Mater. Des. 2018, 139, 363–371. [Google Scholar] [CrossRef]
- Qu, C.; Kinzel, E.C. Infrared metasurfaces created with off-normal incidence microsphere photolithography. Opt. Express 2017, 25, 12632–12639. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Huang, J.; Zeng, Y.; Sun, L.X.; Geng, F.; Liu, H.J.; Wang, F.R.; Jiang, X.D.; Wu, W.D.; Zheng, W.G. Monolayer Colloidal Crystals by Modifified Air-Water Interface Self-Assembly Approach. Nanomaterials 2017, 7, 291. [Google Scholar] [CrossRef] [Green Version]
- Cassagneau, T.; Caruso, F. Inverse Opals for Optical Affinity Biosensing. Adv. Mater. 2002, 14, 1629–1633. [Google Scholar] [CrossRef]
- Martins, T.B.; Burlingame, R.; Mühlen, C.A.V.; Jaskowski, T.D.; Litwin, C.M.; Hill, H.R. Evaluation of Multiplexed Fluorescent Microsphere Immunoassay for Detection of Autoantibodies to Nuclear Antigens. Clin. Diagn. Lab. Immun. 2004, 11, 1054–1059. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.W.; Zhou, Y.; Li, Y.; Hong, M.H. Microsphere enhanced optical imaging and patterning: From physics to applications. Appl. Phys. Rev. 2019, 6, 021304-01–021304-22. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yeo, T.L.; Zhao, W.Z.; Sun, T.; Grattan, K.T.V.; Lade, R.; Powell, B.D.; Foster-Turner, G.; Osborne, M. Development of multi-wavelength microsphere fifibre laser system for potential sensor applications. Opt. Commun. 2009, 282, 401–405. [Google Scholar] [CrossRef]
- Hu, W.T.; Liu, B.C.; Wang, Q.; Liu, Y.; Liu, Y.X.; Jing, P.; Yu, S.L.; Liu, L.X.; Zhang, J. A magnetic double-shell microsphere as a highly effiffifficient reusable catalyst for catalytic applications. Chem. Commun. 2013, 49, 7596–7598. [Google Scholar] [CrossRef]
- Last, A.; Hein, H.; Mohr, J. Shape deviations in masks for optical structures produced by electron beam lithography. Microsyst. Technol. 2004, 10, 527–530. [Google Scholar] [CrossRef]
- Widawski, G.; Rawiso, M.; Francois, B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Collings, D.; Philips, A.; Patel, S. Three-Dimensionally Ordered Array of Air Bubbles in a Polymer Film. Science 2001, 292, 79–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.L.; Sun, L.C.; Zhong, Y.W.; Ma, C.Y.; Li, L.; Xie, S.Y.; Svrcek, V. Fabrication of multi-level carbon nanotube arrays with adjustable patterns. Nanoscale 2012, 4, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.Y.; Zhang, Y.Y.; Kong, J.H.; Zou, C.J.; Li, C.M.; Lu, X.H.; Ma, J.; Boey, F.Y.C.; Chen, X.D. Assembly of Graphene Sheets into Hierarchical Structures for High-Performance Energy Storage. ACS Nano 2011, 5, 3831–3838. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Zhang, L.; Wang, Y.X.; Yang, X.L.; Zhao, N.; Zhang, X.L.; Xu, J. A Bottom-Up Approach To Fabricate Patterned Surfaces with Asymmetrical TiO2 Microparticles Trapped in the Holes of Honeycomblike Polymer Film. J. Am. Chem. Soc. 2011, 133, 3736–3739. [Google Scholar] [CrossRef]
- Sun, W.; Shao, Z.; Ji, J. Particle-assisted fabrication of honeycomb-structured hybrid films via breath figures method. Polymer 2010, 51, 4169–4175. [Google Scholar] [CrossRef]
- Wan, L.S.; Lv, J.; Ke, B.B.; Xu, Z.H. Facilitated and Site-Specifific Assembly of Functional Polystyrene Microspheres on Patterned Porous Films. ACS Appl. Mater. Interfaces 2010, 2, 3759–3765. [Google Scholar] [CrossRef]
- Tamaki, K.; Matsushita, S.; Shimomura, M. Fabrication of polymeric particles composed of two-dimensionally self-assembled nanoparticles by use of a microporous film as a template. Colloids Surf. A 2008, 313–314, 630–635. [Google Scholar] [CrossRef]
- Yabu, H.; Inoue, K.; Shimomura, M. Multiple-periodic structures of self-organized honeycomb-patterned films and polymer nanoparticles hybrids. Colloids Surf. A 2006, 284–285, 301–304. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, M.C.; Zhou, D.; Li, B.; Chen, Z.J.; Zhang, H.; Wu, L.X. Asymmetric surface modification of yeast cells for living self-assembly. Chem. Commun. 2018, 54, 14112–14115. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ma, Y.Y.; Sims, S.; Wu, L.X. A patterned porous polymer film for localized capture of insulin and glucose-responsive release. J. Mater. Chem. B 2015, 3, 1281–1288. [Google Scholar] [CrossRef]
- Liang, J.; Ma, Y.Y.; Sun, H.; Li, W.; Wu, L.X. Polyanion cluster patterning on polymer surface through microemulsion approach for selective adsorption of proteins. J. Colloid Interfaces Sci. 2013, 409, 80–87. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gong, S.; Zhu, J.; Zhang, J.; Liang, J. Mono-Dispersed Microspheres Locally Assembled on Porous Substrates Formed through a Microemulsion Approach. Polymers 2020, 12, 964. https://doi.org/10.3390/polym12040964
Zhang J, Gong S, Zhu J, Zhang J, Liang J. Mono-Dispersed Microspheres Locally Assembled on Porous Substrates Formed through a Microemulsion Approach. Polymers. 2020; 12(4):964. https://doi.org/10.3390/polym12040964
Chicago/Turabian StyleZhang, Jianfeng, Shuxin Gong, Jiahang Zhu, Jiejing Zhang, and Jing Liang. 2020. "Mono-Dispersed Microspheres Locally Assembled on Porous Substrates Formed through a Microemulsion Approach" Polymers 12, no. 4: 964. https://doi.org/10.3390/polym12040964
APA StyleZhang, J., Gong, S., Zhu, J., Zhang, J., & Liang, J. (2020). Mono-Dispersed Microspheres Locally Assembled on Porous Substrates Formed through a Microemulsion Approach. Polymers, 12(4), 964. https://doi.org/10.3390/polym12040964