Preparation of Nanocomposite Alginate Fibers Modified with Titanium Dioxide and Zinc Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
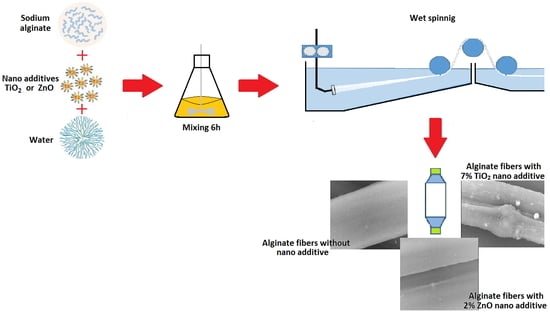
2.2. Methods
3. Results and Discussion
3.1. Infrared Spectroscopy—FTIR
3.2. Degree of Crystallinity—WAXS
3.3. Scanning Electron Microscopy—SEM
3.4. Differential Scanning Calorimetry—DSC
3.5. Porosity
3.6. Mechanical Properties
3.7. Water Sorption at RH60 and Water Retention Value
3.8. Antimicrobial activity studies
- Escherichia coli ATCC 10536 (gram negative rod);
- Staphylococcus aureus ATCC 6538 (gram positive granuloma);
- Aspergillus niger ATCC 16404 (molds).
- N0 – number of microorganisms cultured on a non-woven control after 0 hours;
- Nt – number of microorganisms grown on modified non-woven fabric after 24 h.
- Nt’- number of microorganisms grown on control nonwoven after 24 h;
- Nt - number of microorganisms grown on modified non-woven fabric after 24 h.
- N0—number of microorganisms grown on control nonwoven after 0 h;
- Nt—number of microorganisms grown on modified non-woven fabric after 24 h.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mroczek-Sosnowska, N.; Jaworski, S.; Siennicka, A.; Gondek, A. Unikalne właściwości nanocząstek srebra. Pol. Drob. 2013, 20, 6–8. [Google Scholar]
- Wyrębska, Ł.; Szuster, L.; Stawska, H. Synteza i aplikacja nowych pochodnych wybranych polisacharydów Część I: Przegląd literatury. Technologia i Jakość Wyrobów 2014, 59, 1–16. [Google Scholar]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draczyński, Z.; Buguń, M.; Rabiej, S.; Mikołajczyk, T.; Szparaga, G.; Król, P. New Generation Butyric-Acetate Copolymer of Chitin (BOC) Fibres with Ceramic HAp and TCP Nano additives for the Manufacture of Fibrous Composite Materials. Fiber Polym. 2013, 14, 1107–1117. [Google Scholar] [CrossRef]
- Draczyński, Z.; Buguń, M.; Rabiej, S.; Mikołajczyk, T.; Szparaga, G.; Król, P. The influence of forming conditions on the properties of the fibers made of chitin butyryl-acetic copolyester for medical applications. J. Appl. Polym. Sci. 2012, 127, 3569–3577. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S.I.; Cho, C.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar] [CrossRef]
- 7. Khalil, Saif El Din. Deposition and Structural Formation of 3D Alginate Tissue Scaffolds. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2012.
- Kosmala, K.; Szymańska, R. Nanocząstki tlenku tytanu (IV). Otrzymywanie, właściwości i zastosowanie. Kosmos 2016, 65, 235–245. [Google Scholar]
- Piecyk, L. Nanokompozyty termoplastyczne. Tworzywa Sztuczne i Chemia 2006, 2, 20–25. [Google Scholar]
- Pereira, L.; Sousa, A.; Coelho, H.; Amolo, A.M.; Ribeiro-Claro, P.J.A. Use of FTIR, FT Raman and C – NMR spectroscopy for identification of some seaweed phycocolloids. Biomol. Eng. 2003, 20, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Barikosky, M. Dressing product with a calcium alginate matrix and method of production of the same. U.S. Patent 5,981,821, 9 November 1999. [Google Scholar]
- Fenton, J.C.; Keys, A.F.; Mahoney, P.M.J. Alginate fabric, its use in wound dressing and surgical hemostats and a process for its manufacture. U.S. Patent 5,714,955, 3 February 1998. [Google Scholar]
- Motta, G.J. Calcium alginate topical wound dressings: A new dimension in the cost-effective treatment for exudating dermal wounds and pressure sores. Ostomy Wound Manag. 1989, 25, 52–56. [Google Scholar]
- Polyak, B.; Geresh, S.; Marks, R.S. Synthesis and Characterization of a Biotin-Alginate Conjugate and Its Application in Biosensor Construction. Biomacromolecules 2004, 5, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rabeah, K.; Polyak, B.; Ionescu, R.E.; Cosnier, S.; Marks, R.S. Synthesis and Characterization of a Pyrrole-Alginate Conjugate and Its Application in a Biosensor Construction. Biomacromolecules 2005, 6, 3313–3318. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jones, J.R. Biomaterials, Artificial Organs and Tissue Engineering, 1st ed.; Woodhead Publishing Ltd: Cambridge, UK, 2005; pp. 333–344. [Google Scholar]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Franceschi, R.T.; Mooney, D.J. Cellinteractive Alginate Hydrogels for Bone Tissue Engineering. J. Dent. Res. 2001, 80, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Shachar, M.; Leor, J.; Cohen, S. Cardiac tissue engineering Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305–312. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of Partially Oxidized Alginate and Its Potential Application for Tissue Engineering. J. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Mierisch, C.M.; Cohen, S.B.; Jordan, L.C.; Robertson, P.G.; Balian, G.; Diduch, D.R. Transforming growth factor-ß in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. J. Arthrosc. Relat. Surg. 2002, 18, 892–900. [Google Scholar] [CrossRef]
- Lis, A.; Szarek, D.; Laska, J. Strategie inżynierii biomateriałów dla regeneracji rdzenia kręgowego: Aktualny stan wiedzy. Polim. Med. 2013, 43, 59–80. [Google Scholar]
- Boguń, M. Nanokompozytowe Włókna Alginianowe i Kompozyty z Ich Udziałem do Zastosowań w Inżynierii Biomateriałowej, Zeszyty Naukowe, 1062nd ed.; Lodz University of Technology Publishing House: Lodz, Poland, 2010; pp. 10–20. [Google Scholar]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Pryliński, M.; Limanowska-Shaw, H. Właściwości tytanu i problem nadwrażliwości na ten metal. Implantoprotetyka 2007, 4, 50–52. [Google Scholar]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; Frerichs, S.; Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 2007, 230, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Kafizas, A.; Noor, N.; Carmalt, C.J.; Parkin, I.P. TiO2-based transparent conducting oxides; the search for optimum electrical conductivity using a combinatorial approach. J. Mater. Chem. C 2013, 1, 6335–6346. [Google Scholar] [CrossRef]
- Mazur, M.; Sieradzka, K.; Kaczmarek, D.; Domaradzki, J.; Wojcieszak, D.; Domanowski, P.; Prociów, E. Investigation of physicochemical and tribological properties of transparent oxide semiconducting thin films based on Ti-V oxides. Mater. Sci.-Pol. 2013, 31, 434–445. [Google Scholar] [CrossRef]
- Piegat, A. Synteza i właściwości nanostrukturalnych układów polimerowych dla inżynierii tkankowej. Ph.D. Thesis, West Pomeranian University of Technology, Szczecin, Poland, 2010. [Google Scholar]
- Smith, L.; Kuncic, Z.; Ostrikov, K.; Kumar, S. Nanoparticles in Cancer imaging and therapy. J. Nanomat. 2012, 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Wiatr, E.; Nowakowska, D. Zastosowanie nanocząstek w materiałach stomatolo-gicznych. Protet. Stomatol. 2013, 6, 466–475. [Google Scholar] [CrossRef]
- Shokuhfar, T.; Sinha-Ray, S.; Sukotjo, C.; Yarin, A.L. Interaction of anti-inflammatory drug molecules within TiO2 nanotubes. RSC Adv. 2013, 3, 17380–17386. [Google Scholar] [CrossRef]
- Nejatian, T.; Sultan, Z.; Zafar, M.S.; Najeeb, S.; Shahab, S.; Mazafari, M.; Hopkinson, L.; Sefat, F. Dental Biocomposites. Biomaterials for Oral and Dental Tissue Engineering, 1st ed.; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2017; pp. 65–84. [Google Scholar]
- Żelechowska, K. Nanotechnologia w Praktyce, 1st ed.; PWN: Warsaw, Poland, 2016. [Google Scholar]
- Wachnicki, Ł. Strukturalna, optyczna i elektryczna charakteryzacja warstw monokrystalicznych oraz nanostruktur tlenku cynku otrzymywanych metodą osadzania warstw atomowych. Ph.D. Thesis, Institute of Physics PAN, Warszawa, Poland, 2014. [Google Scholar]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Wang, C.-L.; Ko, H.H.; Hwang, W.-S.; Chang, K.-m.; Li, W.L.; Huang, H.-H.; Chang, Y.-H.; Wang, M.C. Synthesis of zinc oxide nanocrystalline powders for cosmetic applications. Ceram. Int. 2010, 36, 693–698. [Google Scholar] [CrossRef]
- Teterycz, H.; Suchorska-Woźniak, P.; Fiedot, M.; Karbownik, I. Deposition of Zinc Oxide on the Materials Used in Medicine. Fibres Text. East. Eur. 2014, 22, 126–132. [Google Scholar]
- Kubiak, T. Wykorzystanie pokryć z poli(glikolu etylenowego) i chitozanu do zapewnienia biokompatybilności nanocząstkom w aplikacjach biomedycznych. Polim. Med. 2014, 44, 119–127. [Google Scholar] [PubMed]
- Butkus, M.A.; Edling, L.; Labare, M.P. The efficacy of silver as a bactericidal agent: Advantages, limitations and considerations for future use. J. Water Supply Res. Technol. AQUA 2003, 52, 407–416. [Google Scholar] [CrossRef]
- Bojarska, M.; Szwast, M.; Jakubiak, S.; Michalski, J.; Gradoń, L. Membrany polimerowe modyfikowane tlenkiem cynku – metoda wytwarzania. Prosimy cytować jako: Inż. Ap. Chem. 2013, 52, 521–522. [Google Scholar]
- Jantas, R.; Draczyński, Z.; Stawski, D. Starch functionalized by chloroacetate groups: Coupling of bioactive salicylic acid. Starch-Stärke 2007, 59, 366–370. [Google Scholar] [CrossRef]










| Sample | The Degree of Crystallinity (%) |
|---|---|
| Alginate fibers without nano additive | 14.52 |
| Alginate fibers with 2% ZnO | 20.75 |
| Alginate fibers with 7% TiO2 | 39.36 |
| Sample | Total Pore Area (m2/g) | Average Pore Diameter (nm) |
|---|---|---|
| Alginate fibers without nano additive | 0.100 ± 0.005 | 37300 ± 2000 |
| Alginate fibers with 2% ZnO | 0.077 ± 0.005 | 42200 ± 2100 |
| Alginate fibers with 7% TiO2 | 0.055 ± 0.005 | 43500 ± 2120 |
| Sample | Tensile Strength (cN) | Elongation at break (%) | Specific Strength (cN/tex) |
|---|---|---|---|
| Alginate fibers without nano additive | 1106.16 ± 178.46 | 4.39 ± 2.18 | 15.80 ± 2.49 |
| Alginate fibers with 2% ZnO | 1720.18 ± 69.37 | 3.62 ± 0.93 | 18.38 ± 1.68 |
| Alginate fibers with 7% TiO2 | 1147.64 ± 93.80 | 3.15 ± 0.79 | 16.88 ± 1.31 |
| Sample | Sorption RH 60 (%) | Water Retention Value (%) |
|---|---|---|
| Alginate fibers without nano additive | 21.42 | 81.63 |
| Alginate fibers with 2% ZnO | 41.04 | 89.52 |
| Alginate fibers with 7% TiO2 | 30.07 | 95.46 |
| Sample | Type of Microorganism | Reduction (%) | Biostatic Activity | Biocidal Activity |
|---|---|---|---|---|
| Alginate fibers without nano additive | Escherichia coli | - | - | - |
| Staphylococcus aureus | - | - | - | |
| Aspergillus niger | - | - | - | |
| Alginate fibers with 2% ZnO | Escherichia coli | 67.31 | 1.47 | 0.73 |
| Staphylococcus aureus | 18.74 | 0.51 | 0.09 | |
| Aspergillus niger | 82.22 | 0.37 | 0.75 | |
| Alginate fibers with 7% TiO2 | Escherichia coli | - | 0.57 | 0.46 |
| Staphylococcus aureus | - | 0.26 | - | |
| Aspergillus niger | 76.11 | 0.24 | 0.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borkowski, D.; Krucińska, I.; Draczyński, Z. Preparation of Nanocomposite Alginate Fibers Modified with Titanium Dioxide and Zinc Oxide. Polymers 2020, 12, 1040. https://doi.org/10.3390/polym12051040
Borkowski D, Krucińska I, Draczyński Z. Preparation of Nanocomposite Alginate Fibers Modified with Titanium Dioxide and Zinc Oxide. Polymers. 2020; 12(5):1040. https://doi.org/10.3390/polym12051040
Chicago/Turabian StyleBorkowski, Dominik, Izabella Krucińska, and Zbigniew Draczyński. 2020. "Preparation of Nanocomposite Alginate Fibers Modified with Titanium Dioxide and Zinc Oxide" Polymers 12, no. 5: 1040. https://doi.org/10.3390/polym12051040
APA StyleBorkowski, D., Krucińska, I., & Draczyński, Z. (2020). Preparation of Nanocomposite Alginate Fibers Modified with Titanium Dioxide and Zinc Oxide. Polymers, 12(5), 1040. https://doi.org/10.3390/polym12051040