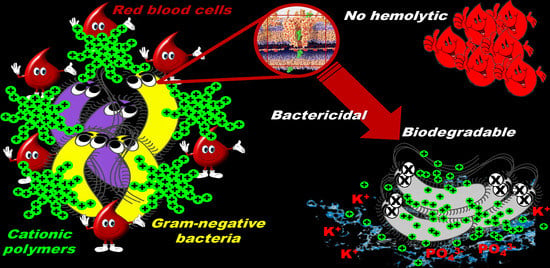
Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review
Abstract
:1. Introduction
2. An Overview on CAMPs, the Template Molecules that Inspired the Development of Cationic Antimicrobial Devices
3. Antimicrobial Polymers
4. Structure of Gram-negative Cells Wall
5. Antimicrobial Cationic Polymers (CAPs) and their Antibacterial “Brute-force Action”
6. Natural Positively Charged Antimicrobial Polymers
6.1. Chitosan
6.2. ε-Polylysine (ε-PL)
7. Synthetic Cationic Antimicrobial Polymers (CAPs)
7.1. Polymers Containing Quaternary Phosphonium and/or Ammonium and/or Guanidinium Groups
7.1.1. Polymers Containing Quaternary Phosphonium and/or Ammonium Groups
7.1.2. Polymers Containing Quaternary Guanidinium Groups
7.2. Polynorborane-based Antimicrobial Polymers
7.3. Polymers Containing not Quaternized Amine Groups
7.4. Polymers Containing Sulfonium Groups
7.5. Polymers Containing Heterocycles with Permanently Cationic Quaternized Nitrogen Atoms
7.6. Quaternized Branched Polyethyleneimine Ammonium Salts
8. Molecular Changes Caused by Cationic Antimicrobial Polymers: A Study Performed
9. Main Factors Influencing the Antimicrobial Properties and Cytotoxic Activities of CAPs
10. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pouch, S.M.; Kubin, C.J.; Satlin, M.J.; Tsapepas, D.S.; Lee, J.R.; Dube, G.; Pereira, M.R. Epidemiology and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteriuria in kidney transplant recipients. Transpl. Infect. Dis. 2015, 17, 800–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaden, J.T.; Li, Y.; Run, F.; Maskarinec, S.A.; Hill-Rorie, J.M.; Wanda, L.C.; Reed, S.D.; Fowler, V.G., Jr. Increased Costs Associated with Bloodstream Infections Caused by Multidrug-Resistant Gram-Negative Bacteria Are Due Primarily to Patients with Hospital-Acquired Infections. Antimicrob. Agents Chemother. 2017, 61, e01709-01716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Antibacterial Agents in Preclinical Development: An Open Access Database; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, e2928. [Google Scholar] [CrossRef]
- Köser, C.U.; Ellington, M.J.; Peacock, S.J. Whole-genome sequencing to control antimicrobial resistance. Trends Genet. 2014, 30, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Leeson, P. Drug discovery: Chemical beauty contest. Nature 2012, 481, 455–456. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, C.; Seo, C.H.; Park, Y. The therapeutic applications of antimicrobial peptides (AMPs): A patent review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clinic. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. New polymeric biocides: Synthesis and antibacterial activities of polycations with pendant biguanide groups. Antimicrob. Agents Chemother. 1984, 26, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gordon, V.D.; Trinkle, D.R.; Schmidt, N.W.; Davis, M.A.; DeVries, C.; Som, A.; Cronan, J.E.; Tew, G.N.; Wong, G.C.L. Mechanism of a prototypical synthetic membrane-active antimicrobial: Efficient hole-punching via interaction with negative intrinsic curvature lipids. Proc. Natl. Acad. Sci. USA 2008, 105, 20595–20600. [Google Scholar] [CrossRef] [Green Version]
- Chin, W.; Yang, C.; Ng, V.W.L.; Huang, Y.; Cheng, J.; Tong, Y.W.; Coady, D.J.; Fan, W.; Hedrick, J.L.; Yang, Y.Y. Biodegradable Broad-Spectrum Antimicrobial Polycarbonates: Investigating the Role of Chemical Structure on Activity and Selectivity. Macromolecules 2013, 46, 8797–8807. [Google Scholar] [CrossRef]
- Gholami, M.; Mohammadi, R.; Arzanlou, M.; Dourbash, F.A.; Kouhsari, E.; Majidi, G.; Mohseni, S.M.; Nazari, S. In vitro antibacterial activity of poly (amidoamine)-G7 dendrimer. BMC Infect. Dis. 2017, 17, e395. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthcare Mater. A 2014, 3, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, e194. [Google Scholar] [CrossRef] [Green Version]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [Green Version]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 57, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.-D.; Won, H.-S.; Kim, J.-H.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial peptides for therapeutic applications: A review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandra, K.M.; Gooderham, W.J.; Hancock, R.E.W. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial Peptides: Interaction With Model and Biological Membranes and Synergism With Chemical Antibiotics. Front Chem. 2018, 6, 204. [Google Scholar] [CrossRef] [Green Version]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Vasil, A.I.; Vasil, M.L.; Hodges, R.S. “Specificity Determinants” Improve Therapeutic Indices of Two Antimicrobial Peptides Piscidin 1 and Dermaseptin S4 against the Gram-negative Pathogens Acinetobacter baumannii and Pseudomonas aeruginosa. Pharmaceuticals 2014, 7, 366–391. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Pogue, J.M.; Ortwine, J.K.; Kaye, K.S. Clinical considerations for optimal use of the polymyxins: A focus on agent selection and dosing. Clin. Microbiol. Infect. 2017, 23, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Spohn, R.; Daruka, L.; Lázár, V.; Martins, A.; Vidovics, F.; Grézal, G.; Méhi, O.; Kintses, B.; Számel, M.; Jangir, P.K.; et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019, 10, e4538. [Google Scholar] [CrossRef]
- Afacan, N.J.; Yeung, A.T.Y.; Pena, O.M.; Hancock, R.E.W. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzic, A.I.; Ioan, S. Concepts, Compounds and the Alternatives of Antibacterials Antibacterial Drugs, 1st ed.; Bobbarala, V., Ed.; Science, Technology and Medicine: London, UK, 2015; pp. 3–28. [Google Scholar]
- Kenawy, E.-R.; Kandil, S. Synthesis, Antimicrobial Activity and Applications of Polymers with Ammonium and Phosphonium Groups. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications, 1st ed.; Muñoz-Bonilla, A., Cerrada, M., Fernández-García, M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 54–74. [Google Scholar]
- Yan, S.; Chen, S.; Gou, X.; Yang, J.; An, J.; Jin, X.; Yang, Y.-W.; Chen, L.; Gao, H. Biodegradable Supramolecular Materials Based on Cationic Polyaspartamides and Pillar[5]arene for Targeting Gram-Positive Bacteria and Mitigating Antimicrobial Resistance. Adv. Funct. Mater. 2019, 29, e1904683. [Google Scholar] [CrossRef]
- Franklin, T.J.; Snow, G.A. Biochemistry and Molecular Biology of Antimicrobial Drug Action, 6th ed.; Springer: New York, NY, USA, 2005; pp. 15–41. [Google Scholar]
- Lambert, P. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J. App. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef]
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.J.; Som, A.; Madkour, A.E.; Eren, T.; Tew, G.N. Infectious disease: Connecting innate immunity to biocidal polymers. Mater. Sci. Eng. R. Rep. 2007, 57, 28–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.H.; Hudson, S.M. Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J. Macromol. Sci. Polym. Rev. 2003, C43, 223–269. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Nonaka, T.; Noda, E.; Kurihara, S. Graft copolymerization of vinyl monomers bearing positive charges or episulfide groups onto loofah fibers and their antibacterial activity. J. App. Polym. Sci. 2000, 77, 1077–1086. [Google Scholar] [CrossRef]
- Li, G.; Shen, J. A study of pyridinium-type functional polymers. IV. Behavioral features of the antibacterial activity of insoluble pyridinium-type polymers. J. App. Polym. Sci. 2000, 78, 676–684. [Google Scholar] [CrossRef]
- Li, G.; Shen, J.; Zhu, Y. A study of pyridinium-type functional polymers. III. Preparation and characterization of insoluble pyridinium-type polymers. J. App. Polym. Sci. 2000, 78, 668–675. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.-J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiller, J.C.; Lee, S.B.; Lewis, K.; Klibanov, A.M. Polymer surfaces derivatized with poly(vinyl-N-hexylpyridinium) kill airborne and waterborne bacteria. Biotechnol. Bioeng. 2002, 79, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, J.; Ichikawa, T.; Ninomiya, S.; Seki, H.; Uohama, K.; Kimura, S.; Yanagimoto, Y.; Barnett, J.W., Jr. Use of ADME studies to confirm the safety of epsilon-polylysine as a preservative in food. Regul. Toxicol. Pharmacol. 2003, 37, 328–340. [Google Scholar] [CrossRef]
- Punia, A.; He, E.; Lee, K.; Banerjee, P.; Yang, N.L. Cationic amphiphilic non-hemolytic polyacrylates with superior antibacterial activity. Chem. Commun. 2014, 50, 7071–7074. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Koski, P. Polyethyleneimine is an effective permeabilizer of Gram-negative bacteria. Microbiol. Microbiol. Soc. 1997, 143, 3193–3199. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Qiu, S.; Lewis, K.; Klibanov, A.M. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol. Prog. 2002, 18, 1082–1086. [Google Scholar] [CrossRef]
- Lin, J.; Qiu, S.; Lewis, K.; Klibanov, A.M. Mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine. Biotechnol. Bioeng. 2003, 83, 168–172. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing n-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Tanfani, F. The n-permethylation of chitosan and the preparation of n-trimethyl chitosan iodide. Carbohydr. Polym. 1985, 5, 297–307. [Google Scholar] [CrossRef]
- Sajomsang, W.; Tantayanon, S.; Tangpasuthadol, V.; Daly, W.H. Quaternization of N-aryl chitosan derivatives: Synthesis, characterization and antibacterial activity. Carbohydr. Res. 2009, 344, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Esin, S.; Benedetti, A.; Maisetta, G.; Fabiano, A.; Zambito, Y.; Batoni, G. Antibacterial, Antibiofilm, and Antiadhesive Properties of Dierent Quaternized Chitosan Derivatives. Int. J. Mol. Sci. 2019, 20, 6297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shi, C.; Liu, Z.; Pan, F.; Meng, R.; Bu, X.; Xing, H.; Deng, Y.; Guo, N.; Yu, L. Antibacterial activity and mode of action of ε-polylysine against Escherichia coli O157:H7. J. Med. Microbiol. 2018, 67, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Matsuoka, H.; Iwamoto, T.; Sakai, H. Antimicrobial action of epsilon-poly-L-lysine. J. Antibiot. 1984, 37, 1449–1455. [Google Scholar] [CrossRef] [Green Version]
- Kito, M.; Onji, Y.; Yoshida, T.; Nagasawa, T. Occurrence of ɛ-poly-L-lysine-degrading enzyme in ε-poly-L-lysine-tolerant Sphingobacterium multivorum OJ10: Purification and characterization. FEMS Microbiol. Lett. 2002, 207, 147–151. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Antimicrobial delivery systems based on electrostatic complexes of cationic ε-polylysine and anionic gum arabic. Food Hydrocoll. 2014, 35, 137–143. [Google Scholar] [CrossRef]
- Ye, R.; Xu, H.; Wan, C.; Peng, S.; Wang, L.; Xu, H.; Aguilar, Z.P.; Xiong, Y.; Zeng, Z.; Wei, H. Antibacterial activity and mechanism of action of ε-poly-L-lysine. Biochem. Biophys. Res. Commun. 2013, 439, 148–153. [Google Scholar] [CrossRef]
- Ikeda, T.; Tazuke, S.; Suzuki, Y. Biologically active polycations 4: Synthesis and antimicrobial activity of poly(trialkylvinylbenzylammonium chloride)s. Makromol. Chem. 1984, 185, 869–876. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef]
- Kougia, E.; Tselepi, M.; Vasilopoulos, G.; Lainioti, G.C.; Koromilas, N.D.; Druvari, D.; Bokias, G.; Vantarakis, A.; Kallitsis, J.K. Evaluation of Antimicrobial Efficiency of New Polymers Comprised by Covalently Attached and/or Electrostatically Bound Bacteriostatic Species, Based on Quaternary Ammonium Compounds. Molecules 2015, 20, 21313–21327. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H. Characterization and antipathogenic evaluation of a novel quaternary phosphonium tripolyacrylamide and elucidation of the inactivation mechanisms. J. Biomed. Mater. Res. Part A 2016, 104A, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Pan, Y.; Xiao, H.; Zhao, Y. Novel quaternary phosphonium-type cationic polyacrylamide and elucidation of dual-functional antibacterial/antiviral activity. RSC Adv. 2014, 4, 46887–46895. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Heine, E.T.; Keul, H.; Möller, M. Multifunctional Poly(Vinyl Amine)s Bearing Azetidinium Groups: One Pot Preparation in Water and Antimicrobial Properties. Macromol. Biosci. 2014, 14, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Gelman, M.A.; Weisblum, B.; Lynn, D.M.; Gellman, S.H. Biocidal activity of polystyrenes that are cationic by virtue of protonation. Org. Lett. 2004, 6, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.; Kuroda, K. Chemical structure of cationic groups in amphiphilic polymethacrylates modulates the antimicrobial and hemolytic activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef]
- Yang, X.; Hu, K.; Hu, G.; Shi, D.; Jiang, Y.; Hui, L.; Zhu, R.; Xie, Y.; Yang, L. Long Hydrophilic-and-Cationic Polymers: A Different Pathway toward Preferential Activity against Bacterial over Mammalian Membranes. Biomacromolecules 2014, 15, 3267–3277. [Google Scholar] [CrossRef]
- Locock, K.E.S.; Michl, T.D.; Valentin, J.D.P.; Vasilev, K.; Hayball, J.D.; Qu, Y.; Traven, A.; Griesser, H.J.; Meagher, L.; Haeussler, M. Guanylated Polymethacrylates: A Class of Potent Antimicrobial Polymers with Low Hemolytic Activity. Biomacromolecules 2013, 14, 4021–4031. [Google Scholar] [CrossRef]
- Gilbert, P.; Pemberton, N.; Wilkinson, D.E. Barrier properties of the Gram-negative cell envelope towards high molecular weight polyhexamethylene biguanides. J. App. Bacteriol. 1990, 69, 585–592. [Google Scholar] [CrossRef]
- Vointseva, I.I.; Gembitsky, P.A. Polyguanidines—Disinfecting Agents and Multifunctional Additives to Composite Materials; LKM-Press: Moscow, Russia, 2009; p. 300. (In Russian) [Google Scholar]
- Broxton, P.; Woodcock, P.M.; Gilbert, P. A study of the antibacterial activity of some polyhexamethylene biguanides towards Escherichia coli ATCC 8739. J. Appl. Bacteriol. 1983, 54, 345–353. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, D.; Guan, Y.; Zheng, A.; Zhong, J.-J. Extensive in vitro activity of guanidine hydrochloride polymer analogs against antibiotics-resistant clinically isolated strains. Mat. Sci. Eng. C 2011, 31, 1836–1843. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Kleshcheva, N.A.; Moroz, A.F.; Didenko, L.V. Secondary and tertiary polydiallylammonium salts: Novel polymers with high antimicrobial activity. Biomacromolecules 2009, 10, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The antimicrobial activity of free and immobilized poly (diallyldimethylammonium) chloride in nanoparticles of poly (methylmethacrylate). J. Nanobiotechnol. 2015, 13, e58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Murata, H.; Koepsel, R.R.; Russell, A.J.; Matyjaszewski, K. Antibacterial polypropylene via surface-initiated atom transfer radical polymerization. Biomacromolecules 2007, 8, 1396–1399. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; De Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, L.D.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Antimicrobial Particles from Cationic Lipid and Polyelectrolytes. Langmuir 2010, 26, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.D.; Carmona-Ribeiro, A.M. Fungicidal nanoparticles of low toxicity from cationic lipid and polyelectrolytes. NSTI Nanotech. 2012, 3, 350–353. [Google Scholar]
- Gabriel, G.J.; Maegerlein, J.A.; Nelson, C.F.; Dabkowski, J.M.; Eren, T.; Nüsslein, K.; Tew, G.N. Comparison of facially amphiphilic versus segregated monomers in the design of antibacterial copolymers. Chem. Eur. J. 2009, 15, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Ilker, M.F.; Nüsslein, K.; Tew, G.N.; Coughlin, E.B. Tuning the Hemolytic and Antibacterial Activities of Amphiphilic Polynorbornene Derivatives. J. Am. Chem. Soc. 2004, 126, 15870–15875. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.J.; Madkour, A.E.; Dabkowski, J.M.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Synthetic mimic of antimicrobial peptide with non-membrane-disrupting antibacterial properties. Biomacromolecules 2008, 9, 2980–2983. [Google Scholar] [CrossRef] [Green Version]
- Eren, T.; Som, A.; Rennie, J.R.; Nelson, C.F.; Urgina, Y.; Nüsslein, K.; Coughlin, E.B.; Tew, G.N. Antibacterial and hemolytic activities of quaternary pyridinium functionalized polynorbornenes. Macromol. Chem. Phys. 2008, 209, 516–524. [Google Scholar] [CrossRef]
- Ng, V.W.L.; Tan, J.P.K.; Leong, J.; Voo, Z.X.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial Polycarbonates: Investigating the Impact of Nitrogen Containing Heterocycles as Quaternizing Agents. Macromolecules 2014, 47, 1285–1291. [Google Scholar] [CrossRef]
- Sellenet, P.H.; Allison, B.; Applegate, B.M.; Youngblood, J.P. Synergistic activity of hydrophilic modification in antibiotic polymers. Biomacromolecules 2007, 8, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.X.; Neoh, K.G.; Cen, L.; Kang, E.T. Antibacterial and antifungal efficacy of surface functionalized polymeric beads in repeated applications. Biotechnol. Bioeng. 2005, 89, 474–484. [Google Scholar] [CrossRef]
- He, Y.; Heine, E.; Keusgen, N.; Keul, H.; Möller, M. Synthesis and characterization of amphiphilic monodisperse compounds and poly(ethylene imine)s: Influence of their microstructures on the antimicrobial properties. Biomacromolecules 2012, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.; Jardine, M.A. Antimicrobial biopolymers. In Advanced Functional Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 493–533. [Google Scholar]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Antimicrobial activity of chitosan nanofibers obtained by electrospinning. Polym. Int. 2011, 60, 1663–1669. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Fonseca, A.C.; Mendonça, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent Developments in Antimicrobial Polymers: A Review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Cheng, H.; Li, J.; Zhang, W.; Shen, Y.; Chen, S.; Ge, Z.; Chen, S. Enhanced water-solubility and antibacterial activity of novel chitosan derivatives modified with quaternary phosphonium salt. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 79–84. [Google Scholar] [CrossRef]
- Wang, C.-H.; Liu, W.-S.; Sun, J.-F.; Hou, G.-G.; Chen, Q.; Cong, W.; Zhao, F. Non-toxic o-quaternized chitosan materials with better water solubility and antimicrobial function. Int. J. Biol. Macromol. 2016, 84, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, M.; Zhou, L.; Tan, H.; Li, J.; Xiao, H.; Li, J.; Snow, J. Synthesis and antibacterial characterization of gemini surfactant monomers and copolymers. Polym. Chem. 2012, 3, 907–913. [Google Scholar] [CrossRef]
- Xiao, H.; Qian, L. Water-Soluble Antimicrobial Polymers for Functional Cellulose Fibres and Hygiene Paper Products. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications, 1st ed.; Muñoz-Bonilla, A., Cerrada, M.L., Fernández-García, M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 75–96. [Google Scholar]
- Gilbert, P.; Mc Bain, A.J. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin. Microbiol. Rev. 2003, 16, 189–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Koepsel, R.R.; Murata, H.; Wu, W.; Lee, S.B.; Kowalewski, T.; Russell, A.J.; Matyjaszewski, K. Nonleaching Antibacterial Glass Surfaces via “Grafting Onto”: The Effect of the Number of Quaternary Ammonium Groups on Biocidal Activity. Langmuir 2008, 24, 6785–6795. [Google Scholar] [CrossRef]
- Saif, M.J.; Anwar, J.; Munawar, M.A. A novel application of quaternary ammonium compounds as antibacterial hybrid coating on glass surfaces. Langmuir 2009, 25, 377–379. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Beyth, N.; Nyska, A.; Weiss, E.I.; Golenser, J.; Domb, A.J. Surface characterization and biocompatibility of restorative resin containing nanoparticles. Biomacromolecules 2008, 9, 3044–3050. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Iatridi, Z.; Moschakou, M.; Damigos, P.; Bokias, G.; Kallitsis, J.K. Development of Cu2+ and/or phosphonium-based polymeric biocidal materials and their potential application in antifouling paints. Prog. Org. Coat. 2012, 75, 190–199. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, J.C.; Tu, Q.; Nie, N.; Sha, J.; Liu, W.M.; Liu, R.; Zhanga, Y.; Wanga, J. Surface modification of PDMS by surface-initiated atom transfer radical polymerization of water-soluble dendronized PEG methacrylate. Colloids Surf. B Biointerfaces 2011, 88, 85–92. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Abdel-Hay, F.I.; El-Shanshoury, A.-E.R.; El-Newehy, M.H. Biologically active polymers. V. Synthesis and antimicrobial activity of modified poly (glycidyl methacrylate-co-2-hydroxyethylmethacrylate) derivatives with quaternary ammonium and phosphonium salts. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2384–2393. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Novel polycationic biocides: Synthesis and antibacterial activity of polymeric phosphonium salts. J. Polym. Sci. A Polym. Chem. 1993, 31, 335–343. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Mendonça, P.V.; Almeida, M.C.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Increasing the Antimicrobial Activity of Amphiphilic Cationic Copolymers by the Facile Synthesis of High Molecular Weight Stars by Supplemental Activator and Reducing Agent Atom Transfer Radical Polymerization. Biomacromolecules 2019, 20, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Zumbuehl, A. Non-leaching surfaces capable of killing microorganisms on contact. J. Mater. Chem. 2009, 19, 7796–7806. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 162, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Xia, Q.; Xiao, H. Cationic Polymers with Tailored Structures for Rendering Polysaccharide-Based Materials Antimicrobial: An Overview. Polymers 2019, 11, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koromilas, N.D.; Lainioti, G.C.; Oikonomou, E.K.; Bokias, G.; Kallitsis, J.K. Synthesis and self-organization in dilute aqueous solution of hydrophobically modified polycations and polyampholytes based on 4-vinyl benzyl chloride. Eur. Polym. J. 2014, 54, 39–51. [Google Scholar] [CrossRef]
- Wang, S.-W.; Liu, W.; Colby, R.H. Counterion dynamics in polyurethane-carboxylate ionomers with ionic liquid counterions. Chem. Mater. 2011, 23, 1862–1873. [Google Scholar] [CrossRef]
- Jangu, C.; Long, T.E. Phosphonium cation-containing polymers: From ionic liquids to polyelectrolytes. Polymer 2014, 55, 3298–3304. [Google Scholar] [CrossRef]
- Broxton, P.; Woodcock, P.M.; Heatley, M.; Gilbert, P. Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli. J. Appl. Bacteriol. 1984, 57, 115–124. [Google Scholar] [CrossRef]
- Lienkamp, K.; Madkour, A.E.; Musante, A.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: A molecular construction kit approach. J. Am. Chem. Soc. 2008, 130, 9836–9843. [Google Scholar] [CrossRef] [Green Version]
- Haney, E.F.; Straus, S.K.; Hancock, R.E.W. Reassessing the Host Defense Peptide Landscape. Front. Chem. 2019, 7, e43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, E.; Sovadinova, I.; Kuroda, K. Structural determinants of antimicrobial activity and biocompatibility in membrane disrupting methacrylamide random copolymers. Biomacromolecules 2009, 10, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.D.; Sampaio, J.L.; Carmona-Ribeiro, A.M. Supramolecular cationic assemblies against multidrug-resistant microorganisms: Activity and mechanism of action. Int. J. Mol. Sci. 2015, 16, 6337–6352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, A.; Ikeda, T.; Endo, T. Antibacterial activity of polymeric sulfonium salts. J. Polym. Sci. A Polym. Chem. 1993, 31, 2873–2876. [Google Scholar] [CrossRef]
- Hirayama, M. The Antimicrobial Activity, Hydrophobicity and Toxicity of Sulfonium Compounds, and Their Relationship. Biocontrol. Sci. 2011, 16, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, B.C.; Applegate, B.M.; Youngblood, J.P. Hemocompatibility of hydrophilic antimicrobial copolymers of alkylated 4-vinylpyridine. Biomacromolecules 2007, 8, 2995–2999. [Google Scholar] [CrossRef]
- Stratton, T.R.; Rickus, J.L.; Youngblood, J.P. In Vitro Biocompatibility Studies of Antibacterial Quaternary Polymers. Biomacromolecules 2009, 10, 2550–2555. [Google Scholar] [CrossRef]
- Davidson, R.L. Water-Soluble Resins, 2nd ed.; Reinhold Book Corp.: New York, NY, USA, 1968; pp. 1–240. [Google Scholar]
- Yaroslavov, A.A.; Melik-Nubarov, N.S.; Menger, F.M. Polymer induced flip-flop in biomembranes. Acc. Chem. Res. 2006, 39, 702–710. [Google Scholar] [CrossRef]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic biocides with pendant active groups: Molecular weight dependence of antibacterial activity. Antimicrob. Agents Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.; Feiertag, P.; Hayn, G.; Saf, R.; Hönig, H. Structure–activity relationships of oligoguanidines—influence of counterion, diamine, and average molecular weight on biocidal activities. Biomacromolecules 2003, 4, 1811–1817. [Google Scholar] [CrossRef]
- Klibanov, A.M. Permanently microbicidal materials coatings. J. Mater. Chem. 2007, 17, 2479–2482. [Google Scholar] [CrossRef]
- Panarin, E.F.; Solovskii, M.V.; Zaikina, N.A.; Afinogenov, G.E. Biological activity of cationic polyelectrolytes. Macromol. Chem. Suppl. 1985, 9, 25–33. [Google Scholar] [CrossRef]
- Lewis, K.; Klibanov, A.M. Surpassing nature: Rational design of sterile-surface materials. Trends Biotechnol. 2005, 23, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Sambhy, V.; Peterson, B.R.; Sen, A. Antibacterial and hemolytic activities of pyridinium polymers as a function of the spatial relationship between the positive charge and the pendant alkyl tail. Angew. Chem. Int. Ed. 2008, 47, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Milovic, N.M.; Wang, J.; Lewis, K.; Klibanov, A.M. Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol. Bioeng. 2005, 90, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.M.; Kleshcheva, N.A.; Vasilieva, Y.A.; Gromova, G.L.; Timofeeva, G.I.; Filatova, M.P. Synthesis of novel polymers based on monomers of the diallylamine series: Mechanistic and kinetic study. Polym. Sci. Ser. A 2005, 47, 273–282. [Google Scholar]
- Glukhov, E.; Stark, M.; Burrows, L.L.; Deber, C.M. Basis for selectivity of cationic antimicrobial peptides for bacterial vs. mammalian membranes. J. Biol. Chem. 2005, 280, 33960–33967. [Google Scholar] [CrossRef] [Green Version]





| Category | Description | Action | Advantages | Drawbacks | |
|---|---|---|---|---|---|
| biocidal polymer | necessarily cationic | quaternary phosphonium | unspecific electrostatic/disruptive interaction with negatively charged bacteria membranes | no presence of toxic biocide no release of harmful biocides for environmental minor trend to develop resistance | hemolytic toxicity fast clearance from circulation high uptake in the reticuloendothelial system |
| guanidinium | |||||
| tertiary sulfonium | |||||
| primary, secondary, tertiary, quaternary ammonium | |||||
| biocidal polymers embodied by the entire macromolecule not requiring biocidal monomers | |||||
| polymeric biocide | from polymerization of antimicrobial monomers unnecessary cationic presence of repeated antimicrobial functionalities | same action of the attached biocide moieties | lower systemic toxicity lower hemolytic toxicity lower clearance | less active than free biocide drugs for steric hindrance cause by polymer | |
| biocide-releasing polymer | unnecessary cationic not intrinsic activity of polymer presence of loaded cleavable antimicrobial drugs covalently linked or by physically entrapped | by releasing the entrapped or bond antimicrobial drugs | target release of biocide higher concentration of biocide at the target site excellent efficacy | significant reduction of activity in time toxicity of free biocide | |
| Family | Genus | Species |
|---|---|---|
| Campylobacteraceae | Campylobacter | Campylobacter coli Campylobacter concisus Campylobacter jejuni Campylobacter rectus |
| Arcobacter | Arcobacter butzleri Arcobacter cryaerophilus | |
| Enterobacteriaceae | Citrobacter | Citrobacter amalonaticus Citrobacter braakii Citrobacter farmeri Citrobacter freundii Citrobacter gillenii Citrobacter koseri |
| Enterobacter | Enterobacter aerogenes Enterobacter agglomerans Enterobacter cloacae Enterobacter cowanii Enterobacter gergoviae | |
| Escherichia | Escherichia coli | |
| Klebsiella | Klebsiella pneumoniae | |
| Morganella | Morganella morganii | |
| Proteus | Proteus vulgaris Proteus mirabilis | |
| Shigella | Shigella dissenteriae | |
| Salmonella | Salmonella tiphy | |
| Yersinia | Yersinia pestis (responsible for the plague) Yersinia pseudotuberculosis Yersinia enterocolitica | |
| Serratia | Serratia marcescens | |
| Aerobacter | Aerobacter aerogenes | |
| Enterobacter | Enterobacter sakazakii | |
| Moraxellaceae | Acinetobacter | Acinetobacter baumannii Acinetobacter beijerinckii Acinetobacter bereziniae Acinetobacter boissieri |
| Moraxella | Moraxella catarrhalis (Branhamella catarrhalis) | |
| Neisseriaceae | Neisseria | Neisseria meningitidis |
| Hemophilus | Hemophilus influenzae | |
| Pasteurellaceae | Pasteurella | Pasteurella multocida |
| Pseudomonadaceae | Pseudomonas | Pseudomonas aeruginosa |
| Vibrionaceae | Vibrio | Vibrio cholerae (responsible for cholera) Vibrio fischeri Stenotrophomonas maltophilia |
| Possible Constituents of the Bacteria Outer Envelope | Gram-Negative Bacteria | Features | Components | |
|---|---|---|---|---|
| inner cell cytoplasmic membrane (CM) | present | negatively charged | phospholipid bilayer functional membrane proteinsenzymes | |
| peptidoglycan layer | present | much thicker that in Gram-positive bacteria | sugars (N-acetylglucosamine, N-acetylmuramic acid) amino acids (tetrapeptides) | |
| outer membrane (OM) | present | high density of negative charges | lipopolysaccharide (outer leaflet) | lipid A |
| polysaccharide core | ||||
| O antigen | ||||
| phospholipids membrane proteins | ||||
| lipoproteins (attached to polysaccharide backbone) | single-layer phospholipid | |||
| hydrophilic proteins | ||||
| porins | pores for particular molecules | |||
| periplasmic space | present | concentrated gel-like substance | periplasm transport proteins sensory proteins peptidoglycan | |
| surface layer (S-layer) | present | directly attached to OM | proteins glycoproteins | |
| flagella | possibly present | four supporting rings instead of two | helical protein flagellin with the shape of a 20-nanometer-thick hollow tube | |
| lipoteichoic acids teichoic acids | absent | molecules that completely cross the wall linked to phospholipids or to peptidoglycan | polyvalent alcohol polymers bonded together through a phosphate group | |
| Braun’s lipoprotein | possibly present | link between the OM and the peptidoglycan chain by a covalent bond | hydrophilic protein hydrophobic lipid head | |
| Step | Small Molecule Antimicrobial Agents | CAPs |
|---|---|---|
| initial absorption | weak | strong |
| diffusion past the cell wall | strong | weak |
| binding into the membrane | weak | strong |
| disruption and disintegration of the membrane | weak | strong |
| Structure of Cationic Polymer | Target Bacteria | Antibacterial Activity Expressed as MIC/NBC (µg/mL) Log Reduction§ Antibacterial Efficiency# | Drawbacks | Advantage | Sectors of Application/Uses |
|---|---|---|---|---|---|
 * *[42,43] | E. coli X. campestris Salmonella enterica S. tiphymurium P. aeruginosa Aeromonas hydrophila Shigella dysenteriae Vibrio cholerae V. parahemolyticus P. fluorescens Enterobacter aerogenes | 20–1000 500 2000–3000 1000–2000 200–1700 1000 > 200 200 150–1000 250–1000 250 | difficult control over structure and properties poor reproducibility of results active only at acidic pH non-tuberculocidal non-sporicidal | biocompatible biodegradable available in a large scale low-cost | agriculture sector packaging textile industry biomedicine |
 [54,55,56,57,58] | P. fluorescens P. aeruginosa E.coli | > 128 150– > 5000 16–64 | difficult control over structure and properties poor reproducibility of results non-tuberculocidal non-sporicidal | biocompatible biodegradable available in a large scale active at every pH value | pharmaceutic biomedical |
 * *[49,59,60,61,62,63] | E. coli K-12 E. coli F-2 E. coli B E. coli P. fluorescens P. putida P. aeruginosa S. marcescens S. fonticola S. typhimurium | 1–10 2 1 1 100 2–100 3–100 8–100 10 10 | difficult control over structure and properties poor reproducibility of results non-tuberculocidal no sporicidal | water soluble biocompatible biodegradable available in a large scale inexpensive low toxicity | food sector antimicrobial food packaging |
 [64,65] | E. coli Aerobacter aerogenes P. aeruginosa | 10–33 10–33 66–100 | activity reduced by organic material as blood incompatibility with soap non-tuberculocidal no sporicidal | chemical stability non-volatility long-term activity lower toxicity that low MW molecules broad spectrum of activity | disinfection of surfaces disinfection in hospital, nursing homes, public places waters and waste waters treatment macromolecular carrier for antibiotics medical device coatings food packaging industry textiles and fibrous materials industry antimicrobial coatings with wide applications |
 [66] | P. aeruginosa E. coli | 1.5§ 4.0–6.5§ | activity reduced by organic material as blood incompatibility with soap non-tuberculocidal non-sporicidal | chemical stability non-volatility long-term activity lower toxicity that low MW molecules broad spectrum of activity | disinfection of non-critical surfaces antimicrobial coatings for surfaces environments medical device coatings food packaging industry |
 [66] | P. aeruginosa E. coli | inactive | limited activity activity reduced by organic material as blood incompatibility with soap non-tuberculocidal non-sporicidal | chemical stability non-volatility long-term activity lower toxicity that low MW molecules | disinfection of non-critical surfaces antimicrobial coatings for surfaces environments medical device coatings food packaging industry |
 [66] | P. aeruginosa E. coli | inactive | limited activity poor activity incompatibility with soap | chemical stability non-volatility lower toxicity that low MW molecules | no practical application |
 [66] | P. aeruginosa E. coli | 2.5–6.1§ 2.9 § | activity reduced by organic material as blood incompatibility with soap non-tuberculocidal non-sporicidal | chemical stability non-volatility long-term activity lower toxicity that low MW molecules broad spectrum of activity | disinfection of non-critical surfaces: antimicrobial coatings for surfaces environments medical device coatings food packaging industry |
 [66] | P. aeruginosa E. coli | 0.8–0.9 § inactive | limited activity poor activity on Gram-positive incompatibility with soap | chemical stability non-volatility lower toxicity that low MW molecules | no practical application |
 [66] | P. aeruginosa E. coli | inactive | limited activity activity reduced by organic material as blood incompatibility with soap | chemical stability non-volatility long-term activity lower toxicity that low MW molecules | disinfection of non-critical surfaces antimicrobial coatings for surfaces environments medical device coatings food packaging industry |
 [66] | P. aeruginosa E. coli | 5.7–6.1§ 5.1 § | activity reduced by organic material as blood incompatibility with soap non-tuberculocidal non-sporicidal | chemical stability non-volatility long-term activity lower toxicity that low MW molecules broad spectrum of activity | disinfection of non-critical surfaces antimicrobial coatings for surfaces environments medical device coatings food packaging industry |
 [67] | E. coli | 3.9–60 | activity reduced by organic material as blood non-tuberculocidal non-sporicidal | dual-functional chemical stability non-volatility long-term activity lower toxicity that low MW molecules broad-spectrum | medical device coatings applications in high-hygiene products applications in implants in pulp and papermaking |
 [68] | E. coli | 75–250 1 | activity reduced by organic material as blood limited antimicrobial activity | dual-functional device antiviral chemical stability non-volatility long-term activity lower toxicity that low MW molecules | flocculant and disinfectant for water clarification and sterilization papermaking industry additive in hygiene products |
 [69,70] | E. coli | 10–100 | activity reduced by organic material as blood incompatibility with soap non-tuberculocidal non-sporicidal | chemical stability non-volatility low hemolytic toxicity | disinfectants medical device coatings food packaging industry antimicrobial coatings with wide applications |
 [71] | E. coli JM109 | > 50 > 50 | no activity in liquid-medium assay | antimicrobial activity in an agar-plate assay | disinfectants waters and waste waters treatment macromolecular carrier for antibiotics medical device coatings food packaging industry textiles and fibrous materials industry antimicrobial coatings with wide applications |
 [71] | E. coli JM109 | 25 12.5 | highly hemolytic melittin toxin mimic not selective not suitable for clinical uses non-tuberculocidal non-sporicidal | high activity biocidal low-cost | disinfection of non-critical surfaces disinfection of hospital nursing homes public places not suitable for clinical uses |
 [72] | E. coli | 8.1–1000 2,3 7.7–8 2,4 | residual cytotoxicity low biocompatibility non-tuberculocidal non-sporicidal | considerable activity chemically stable tunable cytotoxicity low hemolytic toxicity good selectivity possibility of conjugation with other functional groups | food industry hospitals surface coatings that kill bacteria on contact inhibitors of biofouling or biofilm accumulation |
 [69,73] | P. aeruginosa E. coli | 4–8 5 3–4 5 | low biocompatibility hemolytic toxicity poor selectivity non-tuberculocidal non-sporicidal | High activity chemically stable | disinfectants not suitable for clinical applications |
 [69,73] | P. aeruginosa E. coli | 16–32 5 4 5 | non-tuberculocidal non-sporicidal | High activity chemically stable High biocompatibility Low Hemolytic toxicity High selectivity | promising antimicrobials with potential for clinical applications |
 [69,74] | E. coli | > 1500 | poor activity | Low hemolytic toxicity chemical stability non-volatility long-term activity anti-fungal | promising for producing antimicrobial surface for use in biomedical devices |
 [15,65] | E. coli | 40 | limited spectrum of action non-tuberculocidal non-sporicidal | Non-irritative for skin Non-mutagenic Non-cancerogenic chemical stability non-volatility long-term activity good efficacy | disinfection of non-critical surfaces in hospital, nursing homes, public places |
 [65,75,76,77] | E. coli E. coli ATCC 8739 | 0.29–1.25 2 1.5–10 2,5 1.7–4.5 2 1.8–10 2,5 | non-tuberculocidal non-sporicidal | non-irritative for skin non-mutagenic non-cancerogenic chemical stability non-volatility long-term activity high efficacy | Acanthamoeba keratinitis treatment beer glass sanitizers general disinfection food industry swimming pools disinfection |
 [78] | E.coli Klebsiella spp. P. Mirabilis Citrobacter spp. Citrobacter 6 Enterobacter spp. Enterobacter spp. 6 Indole-positive proteae S. marcescens S. marcescens 6 P. aeruginosa wild type P. aeruginosa 7 Acinetobacter spp. Acinetobacter spp.8 S. maltophilia | 2–8 2–8 4–16 2–8 1–4 2–4 2–4 8–16 4–8 16 4–16 8–16 8–16 8–16 2–16 | non-tuberculocidal non-sporicidal | high antibacterial activity broad spectrum of action high activity against drug resistant bacteria bactericidal at low dosage | permanent sterile-surface materials for hospital infection control |
 [79,80] | E.coli ATCC P. aeruginosa ATCC K. pneumoniae ATCC P. mirabilis | 15–125 1,5 125 5 15 5 315 | non-tuberculocidal non-sporicidal | chemical stability non-volatility long-term activity lower toxicity that low MW molecules broad spectrum of activity | for use in areas of medicine means to fight infection food industry prevention of bacterial contamination water sanitation |
 [79,80,81] [79,80,81] | E.coli ATCC P. aeruginosa ATCC K. pneumoniae ATCC 1 P. mirabilis | 7–62 1,5 31 5 62 5 31 5 | non-tuberculocidal non-sporicidal | chemical stability non-volatility long-term activity lower toxicity that low MW molecules broad spectrum of activity | for use in areas of medicine means to fight infection food industry prevention of bacterial contamination water sanitation |
 [80,81,82,83,84] | E. coli ATCC P. aeruginosa P. aeruginosa MDR K. pneumoniae KPC+ | 5 5 1–2 5 1.5 9 0.9 9 | non-tuberculocidal non-sporicidal | no hemolytic activity high selectivity biocidal activity chemical stability non-volatility long-term activity low susceptibility to resistance broad spectrum of activity | for use in areas of medicine means to fight infection food industry prevention of bacterial contamination water sanitation vegetable oils purification selective removal of free fatty acids from oils and fats |
 [85] | E. coli | 400 | poor activity | high selectivity low hemolytic toxicity | not signaled |
 [86] | E. coli S. marcescens | 25 25 | low selectivity high hemolytic toxicity non-tuberculocidal non-sporicidal | high ability in inserting in CM high ability in disrupting CM high effectiveness | potential antimicrobial agents with low clinical applicability |
 [87] | E. coli S. marcescens | 6 50 | non-tuberculocidal non-sporicidal | bactericidal broad spectrum high ability in inserting in CM high ability in disrupting CM high activity low hemolytic toxicity high selectivity | biomedicine disinfection of cardiovascular implants orthopedic implants |
 [85] | E. coli | 50 | less active than magainin (AMP) considerable residual hemolytic toxicity Poor selectivity non-tuberculocidal non-sporicidal | tunable activity tunable cytotoxicity tunable selectivity depending on HLB | potential antimicrobial agents |
 [88] | E. coli | 4–200 | high cytotoxicity for achieving good activity non-tuberculocidal non-sporicidal | tunable activity tunable selectivity | potential antimicrobial agents with low clinical applicability |
 [69,89] | E. coli P. aeruginosa | 31–63 31–125 | poor activity against fungi poor activity against P. aeruginosa non-tuberculocidal non-sporicidal | broad spectrum good activity on E. coli low hemolytic toxicity high selectivity | development of antimicrobial agents for clinical applications |
 [69,90] | E. coli | 5–70 5,10 | biocompatibility depending on VP content hemolytic toxicity depending on VP content ineffective if highly biocompatible non-tuberculocidal non-sporicidal | tunable biocompatibility tunable hemolytic toxicity acceptable bactericidal activity water-solubility good wettability | permanent bactericidal-surface materials for hospital infection control antimicrobial coatings |
 [91] | E. coli | 8 | non-tuberculocidal non-sporicidal | tunable bactericidal activity low susceptibility to arise resistance high stability reusability | sterile-surface materials to kill air- and waterborne pathogens permanent bactericidal-surface materials for controlling hospital infection antimicrobial coatings antimicrobial beads |
 [52,53] | E. coli P. aeruginosa | 74–96 (glass) 70–95 (NPs) 99 (cotton) 98 (wool) 99 (nylon) 96 (polyester) 73–97 (glass) 67–96 (NPs) 98 (cotton) 97 (wool) 98 (nylon) 98 (polyester) | non-tuberculocidal non-sporicidal | significant to total bactericidal activity no toxicity no release of LPS reusable after washing | permanent bactericidal-surface materials for hospital infection control antimicrobial coatings food industry prevention of bacterial contamination water sanitation antibacterial paints and fillers textile industry |
 [92] | E. coli | 60–100 | non-tuberculocidal no sporicidal poor activity high hemolytic toxicity poor solubility | chemical stability non-volatility | not suitable for clinical applications poor applicability |
| Type of CAPs | MW (Da) | Alkylation | Antimicrobial Activity |
|---|---|---|---|
| oligomeric guanidinium | ↓MW | ↓activity [130] | |
| quaternized N-hexyl-PVP, immobilized on a surface | 160,000 | full bactericidal effect [131] | |
| quaternized N-hexyl, N-methyl-PEI immobilized on a surface | 25,000 | full bactericidal effect [131] | |
| quaternized poly(2-(dimethylamino)ethyl methacrylate) (PDMEMA) | Mn >10,000 | 100% killing efficiency [110] | |
| secondary and tertiary polydiallylamines (PDAAs) | ↑MW | ↓MIC100 [79] | |
| poly alkyldimethyl(vinylbenzyl) ammonium chloride | alkyl C12 chain | ↑activity [64] | |
| cationic quaternary copolymers (vinylamine, aminoalkyl methacrylates, and N-vinyl pyrrolidone) with pendent quaternary ammonium groups | any length | not influenced by the length of the alkyl substituents at the nitrogen [132] | |
| alkylated quaternized PVP polymers | alkyl > C6 chain | ↓activity [133] | |
| quaternized poly (4-vinyl pyridine) (P4VP)-poly (vinylidene fluoride) (PVDF) co-polymer | C4>chain< C10 | ↑activity [134] | |
| quaternized alkyl pyridinium polyoxanorbornene | Chain < C4 | minimal activity [88] | |
| quaternized alkyl pyridinium polyoxanorbornene | Chain > C6 | ↑activity [88] |
| Type of CAP | CAP Structural Properties | Hemolysis (H) Cytotoxicity (C) Biocompatibility (B) | Antimicrobial Activity |
|---|---|---|---|
| quaternized pyridinium–methacrylate copolymers | charge spatially separates by alkyl tails | ↑H ↑C | ↑ [134] |
| hydrophilic vinylpyridine-based co-polymer quaternized with PVP links | use of strongly hydrophilic comonomers (HEMA or PEGMA) | ↓H,C | ↑ [90] |
| quaternized PVP | use of hydrophilic comonomers | ↓H ↑B | ↑ [125] |
| N-hexyl, methyl-PEI | larger size | no appreciable | ↑ [135] |
| polystyrene-based ammonium polymers | protonated tertiary amine groups | not available | ↑ [71] |
| polystyrene-based ammonium polymers | quaternized ammonium groups | not available | ↓ [71] 1 |
| random amphiphilic methacrylamide-based ammonium copolymers | protonated primary amine groups hydrophobic alkyl groups in the side chains | minimal | ↑ [72] |
| random amphiphilic methacrylamide-based ammonium copolymers | protonated tertiary amine groups hydrophobic alkyl groups in the side chains | minimal | ↑ [72] |
| random amphiphilic methacrylamide-based ammonium copolymers | quaternized ammonium groups hydrophobic alkyl groups in the side chains | considerable | ↓ [72] |
| Water-soluble cationic PDAAs containing pyrrolidine links | protonated secondary or tertiary amine groups | not available | ↑ [136] |
| quaternized ammonium groups | quaternized ammonium groups | not available | ↓ [79] |
| poly(diallylammonium trifluoroacetate) (PDAATFA) | protonated secondary amine groups | not available | ↑ [79] |
| poly(diallylammonium trifluoroacetate) (PQAS TFA) | quaternary ammonium groups | not available | ↓ [79] 1 |
| quaternized alkyl pyridinium polyoxanorbornene | chain < C4 | low | ↓ [88] |
| quaternized alkyl pyridinium polyoxanorbornene | chain > C6 | considerable | ↑ [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Schito, A.M. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers 2020, 12, 1195. https://doi.org/10.3390/polym12051195
Alfei S, Schito AM. Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers. 2020; 12(5):1195. https://doi.org/10.3390/polym12051195
Chicago/Turabian StyleAlfei, Silvana, and Anna Maria Schito. 2020. "Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review" Polymers 12, no. 5: 1195. https://doi.org/10.3390/polym12051195
APA StyleAlfei, S., & Schito, A. M. (2020). Positively Charged Polymers as Promising Devices against Multidrug Resistant Gram-Negative Bacteria: A Review. Polymers, 12(5), 1195. https://doi.org/10.3390/polym12051195